
��1�����Թ������һС��ͭƬ��ע��һ�������Ũ���ᣬ���Թܼ��ȣ�ʹ֮��Ӧ����ͭƬ����ʣ�������Ƿ��������?
�ٽ��ۼ������ǣ�_________________________________________��
����100����18 mol/L��H2SO4��Һ�м���������ͭƬ�����ȣ���ַ�Ӧ����ԭ��H2SO4�����ʵ���
A��С��0��9 mol B������0��9 mol C����0��9 mol��1.8 mol֮�� D������1��8 mol
��50g�ĵ������ȵ�100��ʧȥ���ֽᾧˮ��������Ϊ35.6g����ʧȥˮ�������ͭ����Ļ�ѧʽ��
A��CuSO4��H2O B��CuSO4��2H2O C��CuSO4��3H2O D��CuSO4
��2��������пͶ�뵽һ������Ũ�����У���ַ�Ӧ���ռ���SO2��H222.4L(��״��)��
�ټ����ܲ���H2��ԭ����____________ __________________
�������仯������Ũ����ǿ�����Եķ�Ӧ����ʽ�ǣ�
�۷�Ӧ�����Ľ���п������Ϊ_________g��
�����������ܷ��������ĵ���������ʵ����� _ (��ܡ����ܡ�)
(12��)��1���ٷ��淴Ӧ���У�����Ũ�Ȳ��Ͻ��ͣ�ϡ������ͭ����Ӧ�������������ʣ�� ��A ��A
(2)���淴Ӧ�IJ��Ͻ��У�Ũ����Ũ�Ȳ��Ͻ��ͣ���пϡ���ᷴӦ����������
��Zn+2H2SO4(Ũ)��ZnSO4+SO2��+2H2O ��65 �ܲ���
��������
�����������1���������淴Ӧ���У�����Ũ�Ȳ��Ͻ��ͣ�ϡ������ͭ����Ӧ�������������ʣ�ࡣ
��Ũ��������ʵ�����1.8mol�����Ը��ݷ�ӦʽCu��2H2SO4(Ũ) CuSO4��2H2O��SO2��������Ϣٿ�֪��ʵ�ʱ���ԭ��������ʵ���С��0.9mol����ѡA��
CuSO4��2H2O��SO2��������Ϣٿ�֪��ʵ�ʱ���ԭ��������ʵ���С��0.9mol����ѡA��
��50g�ĵ������ȵ�100��ʧȥ���ֽᾧˮ��������Ϊ35.6g����ʧȥ��ˮ��������50g��35.6g��14.4g�����ʵ�����0.8mol�����������ʵ�����0.2mol�����ƺ���1molˮ����˵����Ӧ���е�ˮ�����ʵ���ˮ0.2mol�����Ի�����Ļ�ѧʽ��CuSO4��H2O����ѡA��
��2���������淴Ӧ�IJ��Ͻ��У�Ũ����Ũ�Ȳ��Ͻ��ͣ���пϡ���ᷴӦ������������
������Ũ����ǿ�����Եķ�Ӧ����ʽ��Zn+2H2SO4(Ũ)��ZnSO4+SO2��+2H2O��
�ۻ���������ʵ�����1mol�����ڲ���������������SO2���ڷ�Ӧ�ж���ת��2�����ӵģ����Թ��Ƶ��ӵ�ʧ�غ��֪���μӷ�Ӧ��п�����ʵ���Ҳ��1mol��������65g��
�����ڲ���ȷ��SO2�����ʵ�����������ȷ�����ĵ���������ʵ�����
���㣺����Ũ���������ͭ�Լ�п��Ӧ���йؼ�����ж�
�����������Ǹ߿��еij������ͣ������ۺ���ǿ���������С�������ע�ضԻ�����֪ʶ������ѵ����ͬʱ����Ҫ�Dz�������ѧ���Ľ��ⷽ���뼼�ɣ������ڵ���ѧ����ѧϰ��Ȥ������ѧ������֪��������Ĺؼ�������Ũ����Ļ�ѧ���ʣ��Լ����ú��غ㷨�ڻ�ѧ�����е���ҪӦ�á�


 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Թ������һ��ͭƬ��ע��һ�������Ũ���ᣬ���Թܼ��ȣ�ʹ֮��Ӧ��
��1����ͭƬ����ʣ�࣬����________������ϣ���ѡ��� ��û�С�������������___________________________________________________________________��
��2����һ�����ķ�ͭм��ȡ�����������ַ�����һ����ȡCu��ŨH2SO4ֱ�ӷ�Ӧ�������Ƚ�ͭ�ڿ����м���ʹ֮����CuO������ϡH2SO4��Ӧ������_________�ַ����ã������� _____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꼪��ʡ����һ�и߶�4���¿���ѧ�Ծ����������� ���ͣ������
��.��ͼ��ʾ���ڴ��Թ������һ�ι���������������״
����˿�����Թܵ�����ˮ�У������װ����������Լһ�ܺ۲�
����˿�����ı仯��______________________��ԭ����
____________________���Թ����ˮ������������������߶�ԼΪ
________��ԭ����_________________________________________
________________________________________________________________________.
��.A��B��C�����ձ��зֱ�ʢ����ͬ���ʵ���Ũ�ȵ�ϡ���ᣮ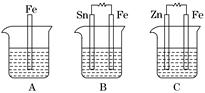
(1)A�з�Ӧ�����ӷ���ʽΪ__________________________________________��
(2)B��Sn�缫�ĵ缫��ӦʽΪ________________________________________��
Sn�缫������Һ��pH__________(���������С�����䡱)��
(3)C�б���ʴ�Ľ�����________���ܷ�ӦʽΪ________________________���Ƚ�
A��B��C��������ʴ�����ʣ��ɿ쵽����˳����______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꼪��ʡ����һ�и߶�4���¿���ѧ�Ծ����������� ���ͣ������
��.��ͼ��ʾ���ڴ��Թ������һ�ι���������������״����˿�����Թܵ�����ˮ�У������װ����������Լһ�ܺ۲쵽��˿�����ı仯��______________________��ԭ����____________________���Թ����ˮ������������������߶�ԼΪ_______��ԭ����_________________________________________________________________________________________________________________.
��.A��B��C�����ձ��зֱ�ʢ����ͬ���ʵ���Ũ�ȵ�ϡ���ᣮ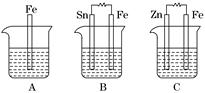
(1)A�з�Ӧ�����ӷ���ʽΪ__________________________________________��
(2)B��Sn�缫�ĵ缫��ӦʽΪ________________________________________��Sn�缫������Һ��pH__________(���������С�����䡱)��
(3)C�б���ʴ�Ľ�����________���ܷ�ӦʽΪ________________________���Ƚ�A��B��C��������ʴ�����ʣ��ɿ쵽����˳����______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�����и�һ��ѧ�ڵ�һ��������⻯ѧ�Ծ� ���ͣ������
���Թ������һ��ͭƬ��ע��һ�������Ũ���ᣬ���Թܼ��ȣ�ʹ֮��Ӧ��
��1����ͭƬ����ʣ�࣬����________������ϣ���ѡ��� ��û�С�������������___________________________________________________________________��
��2����һ�����ķ�ͭм��ȡ�����������ַ�����һ����ȡCu��ŨH2SO4ֱ�ӷ�Ӧ�������Ƚ�ͭ�ڿ����м���ʹ֮����CuO������ϡH2SO4��Ӧ������_________�ַ����ã������� _____________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com