
ij��ɫ����Һ������Ӧ�ų�����������Һ�п��ܺ���Mg2����Cu2����Ba2����H����Ag����SO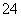 ��SO
��SO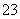 ��HCO
��HCO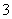 ��OH����NO
��OH����NO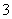 ʮ�������е������֣������ƶ���ȷ���� (����)
ʮ�������е������֣������ƶ���ȷ���� (����)
A������Һ����Al3������ʱ����Һ�п��ܴ��ڣ�SO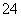 ��NO
��NO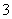 ��H����Mg2��
��H����Mg2��
B������Һ����Al3������ʱ����Һ��һ�����ڣ�H����SO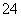 �����ܴ���Mg2��
�����ܴ���Mg2��
C������Һ����[Al(OH)4]������ʱ����Һ��һ�����ڣ�OH����Ba2����NO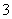
D������Һ����[Al(OH)4]������ʱ����Һ�п��ܴ��ڣ�OH����Ba2����NO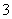 ��SO
��SO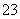
�𰸡�B
�����������⣬һ��������Cu2����HCO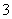 ������Һ����Al3������ʱ��ԭ��Һ�к���H����������NO
������Һ����Al3������ʱ��ԭ��Һ�к���H����������NO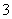 (����������ΪNO)��SO
(����������ΪNO)��SO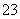 ��OH�����ݵ�����ԭ��֪���бض�����SO
��OH�����ݵ�����ԭ��֪���бض�����SO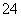 �����Dz��ܴ���Ba2����Ag�������ܺ���Mg2����A�����B����ȷ������Һ����[Al(OH)4]������ʱ��ԭ��Һ����OH�����϶�û��H����Ag����Mg2�����ݵ�����ԭ��֪���бض�����Ba2�������Dz��ܴ���SO
�����Dz��ܴ���Ba2����Ag�������ܺ���Mg2����A�����B����ȷ������Һ����[Al(OH)4]������ʱ��ԭ��Һ����OH�����϶�û��H����Ag����Mg2�����ݵ�����ԭ��֪���бض�����Ba2�������Dz��ܴ���SO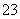 ��SO
��SO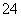 �����ܺ���NO
�����ܺ���NO ��C��D�����
��C��D�����


 �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д� �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�Ԫ�ص����ʼ���ݱ������ȷ����
A.ͬ���ڽ���Ԫ�صĻ��ϼ�Խ�ߣ���ԭ��ʧ��������Խǿ
B.�ڶ�����Ԫ�ش����ң�������۴�+1������+7
C.ͬ����Ԫ�صļ������ӻ�ԭ��Խǿ�����Ԫ�ض�Ӧ����̬�⻯���ȶ���Խǿ
D.IA����VIIA��Ԫ�ؼ���γɹ��ۻ���������ӻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ȼ��ʵ��ʱ��������ǯ��סһС���������ھƾ����ϼ��������ۻ����ῴ���������������ۻ���ʧȥ�����ۻ������������䡣ԭ����____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������������ͬ����������Ӧ���ų������������������ʵ������ٵ��� (����)
A������������Һ B��ϡ����
C������ D��ϡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͬ�������������ʷֱ����Ũ�ȵ�NaOH��Һ��Ӧ������ϵ�о��������ʣ����ļ��������� (����)
A��Al B��Al(OH)3
C��AlCl3 D��Al2O3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
þ�ڿ�����ȼ�ճ�����MgO�⣬����������Mg3N2��ijУ��ѧ��ȤС���ͬѧ����þ�ڿ�����ȼ�պ�Ĺ���(��������)����ʵ�飬̽������ɡ�
(1)����ͬѧȡһ����ȼ�պ�Ĺ���Ͷ��ˮ�У��õ���һ����ʹʪ��ĺ�ɫ ʯ����ֽ���������壬������Ļ�ѧʽΪ______________________________��˵�������к���Mg3N2�����ɸ�����Ļ�ѧ��Ӧ����ʽΪ______________________________________________
ʯ����ֽ���������壬������Ļ�ѧʽΪ______________________________��˵�������к���Mg3N2�����ɸ�����Ļ�ѧ��Ӧ����ʽΪ______________________________________________
________________________________________________________________________��
(2)����ͬѧΪ�ⶨMg3N2�ĺ���������ͼ��ʾװ�ý���ʵ�飬��ַ�Ӧ���ټ���A������Ũ�����������__________________________________________________________
__________________________����A���ȵ�Ŀ����____________________________��
��֪����Ĺ�������Ϊ4.0 g������Cװ������a g��������к�Mg3N2______ g(�ú�a��ʽ�ӱ�ʾ)��
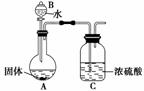
(3)�����е�ͬѧ��Ϊ����ͬѧ�IJⶨ���ƫ�ߣ�������
________________________________________________________________________
________________________________________________________________________��
�е�ͬѧ��Ϊ����ͬѧ�IJⶨ���ƫ�ͣ�������______________________________
________________________________________________________________________��
����ͬѧ�����˸Ľ������ǽ�����ͬѧʵ���еõ������ܹ�����й��ˡ�ϴ�ӡ���������չ��������أ����������Ϊ4.08 g�����������У�ϴ�ӳ����IJ�����________________________________________________________________________
________________________________________________________________________��
þ�ڿ�����ȼ�պ����ɵĹ�����Mg3N2����������Ϊ______________��
(4)��һ��������뺬þ��ʯ���������ȡ����þ�ķ�������������з������ڵ�ԭ�ϳɱ��ߡ���ĿͶ�ʴ��ܺĸߡ�����Ʒ�����õ����⣬��ԭ���ǽ���þ��ʯ��(������þ)����λ�ϣ��������ա�ˮ�ܡ����ˣ��õ���þ����Һ�����������ղ����İ���д���ù���(NH4)2SO4�뺬þ��ʯ�ۻ������ʱ�Ļ�ѧ��Ӧ����ʽ________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(NAΪ�����ӵ�����) (�� ��)
A��124 g P4����P��P���ĸ���Ϊ4NA
B��12 gʯī�к���C��C���ĸ���Ϊ3NA
C��12 g���ʯ�к���C��C���ĸ���Ϊ2NA
D��60gSiO2�к�Si��O���ĸ���Ϊ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��4 mol A�����2 mol B������2 L���ܱ������л�ϲ���һ�������·������·�Ӧ��2A(g)��B(g) 2C(g)����H��0��4 s��Ӧ�ﵽƽ��״̬����ʱ���C��Ũ��Ϊ0.6mol/L������˵������ȷ����(����)
2C(g)����H��0��4 s��Ӧ�ﵽƽ��״̬����ʱ���C��Ũ��Ϊ0.6mol/L������˵������ȷ����(����)
A����Ӧ�����У���A��B��C�����ʵ���Ũ��֮��Ϊ2��1��2ʱ����Ӧ���ﵽƽ��״̬
B��4 s��������B��ʾ�ķ�Ӧ����Ϊ0.075 mol/(L��s)
C����ƽ���������ѹǿ��A��ת���ʽ���
D����ƽ����������¶ȣ�ƽ�ⳣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����л���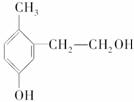 ������˵������ȷ����(����)
������˵������ȷ����(����)
A�����DZ��ӵ�ͬϵ��
B��1 mol���л���������ˮ��Ӧ����2 mol Br2����ȡ����Ӧ
C��1 mol���л�����������Ʒ�Ӧ����0.5 mol H2
D��1 mol���л�������2 mol NaOH��Ӧ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com