
�����������һ����Ҫ�Ļ�����Ʒ��ij��ȤС�����Ʊ���������ƾ���(Na2S2O3��5H2O)��
��.���������ϡ�
(1)Na2S2O3��5H2O����ɫ�����壬������ˮ����ϡ��Һ��BaCl2��Һ����������ɡ�
(2)��Na2CO3��Na2S�����Һ��ͨ��SO2���Ƶ�Na2S2O3�����ò�Ʒ����������Na2SO3��Na2SO4��
(3)Na2SO3�ױ�������BaSO3������ˮ��������ϡ���ᡣ
��.���Ʊ���Ʒ��
ʵ��װ����ͼ��ʾ(ʡ�Լг�װ��)��
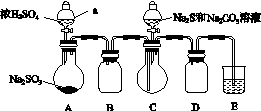
ʵ�鲽�裺
(1)���װ�������ԣ���ͼʾ�����Լ���
����a��������________��E�е��Լ���________(ѡ��������ĸ���)��
A��ϡH2SO4
B��NaOH��Һ
C������NaHSO3��Һ
(2)����C����ƿ����Na2S��Na2CO3�Ļ����Һ������A����ƿ�μ�ŨH2SO4��
(3)��Na2S��Na2CO3��ȫ���ĺ�����Ӧ������C�еĻ�����Һ��________(��д��������)���ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
��.��̽���뷴˼��
(1)Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4����С�����������ʵ�鷽�����뽫��������������(�����Լ���ϡHNO3��ϡH2SO4��ϡ���ᡢ����ˮ��ѡ��)
ȡ������Ʒ���ϡ��Һ���μ�����BaCl2��Һ���а�ɫ�������ɣ�____________________________________��������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��
(2)Ϊ����װ��C������Na2SO4�������ڲ��ı�ԭ��װ�õĻ����϶�ʵ�鲽��(2)�����˸Ľ����Ľ���IJ�����______________________________________________________________________________��
(3)Na2S2O3��5H2O���ܽ�����¶����������������ò�Ʒͨ��________�����ᴿ��
��.(1)�ٷ�Һ©������B
(3)����
��.(1)���ˣ�������ˮϴ�ӳ�����������м�������ϡ���ᡡ(2)����A����ƿ�μ�ŨH2SO4�����������彫װ���еĿ����ž�������C����ƿ����Na2S��Na2CO3�����Һ
(3)�ؽᾧ
[����] ��.(1)��aΪ��Һ©������E���Լ���������SO2β������ΪNaOH��Һ��(3)����Na2S2O3��5H2O����ɫ�����壬������ˮ����Ҫ����Һ�еõ���������ƾ��壬�辭������Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ�����õ���Ʒ����. (1)Na2S2O3��5H2O��ϡ��Һ��BaCl2����������ɣ���ʵ��������а�ɫ�������ɣ������һ����֤�������ɫ�����еμ�ϡ���ᣬ������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��(2)Na2SO3�����ױ������е�����������Na2SO4��Ϊ�˼���Na2SO4���ɵ��������ſ�װ���еĿ�����(3)Na2S2O3��5H2O���ܽ�����¶������������ᾧʱ���������������ʣ����ͨ���ؽᾧ�ķ����ᴿ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ����ᡢ����������ء��������������ʵ�Ľ�����ȷ����(����)
| ѡ�� | �������ʵ | ���� |
| A. | ���ȵĴ�����Һϴȥ���� | Na2CO3��ֱ�������۷�Ӧ |
| B. | Դ���ڿ����о��ñ��� | Ư���е�CaCl2������е�CO2��Ӧ����CaCO3 |
| C. | ʩ��ʱ����ľ��(��Ч�ɷ�ΪK2CO3)������NH4Cl���ʹ�� | K2CO3��HN4Cl��Ӧ���ɰ����ή�ͷ�Ч |
| D. | FeCl3��Һ������ͭ��ӡˢ��·������ | FeCl3�ܴӺ�Cu2������Һ���û���ͭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ȼ�����Ʒ�к�������̼��ء�����غͲ�����ˮ�����ʡ�Ϊ���ᴿ�Ȼ��أ��Ƚ���Ʒ��������ˮ�У���ֽ������ˣ��ڽ���Һ����ͼ��ʾ������в�����
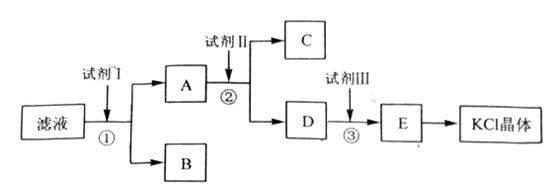
�ش��������⣺
��ʼ��Һ��pH_____________7������ڡ�����С�ڡ����ڡ�������ԭ����_________________________________________________��
�Լ�I�Ļ�ѧʽΪ______________________�����з�����Ӧ�����ӷ���ʽΪ____________________________________________��
�Լ���Ļ�ѧʽΪ______________________�����м����Լ����Ŀ����__________________________________________________________________��
�Լ����������______________________�����з�����Ӧ�����ӷ���ʽΪ__________________________________________________________________��
ijͬѧ��ȡ�ᴿ�IJ�Ʒ0.7759g���ܽ������100mL����ƿ�У�ÿ��ȡ25.00mL��Һ����0.1000mol��L-1������������Һ�ζ������εζ����ı���Һ��ƽ�����Ϊ25.62mL���ò�Ʒ�Ĵ���Ϊ_______________________________________ _____������ʽ����������
_____������ʽ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NA��ʾ����٤������������������ȷ����(����)
A��1 mol FeI2������������Ӧʱת�Ƶĵ�����Ϊ2NA
B��2 L 0.5 mol��L��1�������Һ�����������������ΪNA
C��1 mol Na2O2�����к���������Ϊ4NA
D����ϩ�ͻ�������ɵ�42 g�����������ԭ�ӵĸ���Ϊ6NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ�����Ʊ���������ʱ�����÷�����ȷ����(����)
A��������ʱ����Na2O2��H2O2����Ӧ���ѡ����ͬ�����巢��װ��
B��������ʱ���ñ���NaHCO3��Һ��Ũ���Ά������
C������ϩʱ������ˮ���������ſ������ռ�����
D���ƶ�������ʱ����ˮ��NaOH��Һ����β��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ⱦ�����Чȥ������Դ�ij�������ǻ�ѧ�츣�������Ҫ�о����⡣ij�о�С���������̿�(��Ҫ�ɷ�ΪMnO2��������������������ͭ�����Ƚ���������)���������ͨ�����¼����̼��ѳ�ȼúβ���е�SO2�����Ƶõ�ز���MnO2(��Ӧ������ʡ��)��
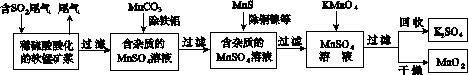
��ش��������⣺
(1)������������ʵ����____(ѡ��������ĸ���)��
A����������ۺ�����
B����ɫ��Ⱦ�ļ���
C������ļ���
(2)��MnCO3�ܳ�ȥ��Һ�е�Al3����Fe3������ԭ����________________________________��
(3)��֪��25 �桢101 kPaʱ��
Mn(s)��O2(g)===MnO2(s)����H����520 kJ/mol
S(s)��O2(g)===SO2(g)����H����297 kJ/mol
Mn(s)��S(s)��2O2(g)===MnSO4(s)��
��H����1065 kJ/mol
SO2��MnO2��Ӧ������ˮMnSO4���Ȼ�����ʽ��____________________________________��
(4)MnO2�����������������ϡ��ö��Ե缫���MnSO4��Һ���Ƶ�MnO2���������ĵ缫��Ӧʽ��________________________________��
(5)MnO2�Ǽ���п�̵�ص��������ϡ�����п�̵�طŵ�ʱ�������ĵ缫��Ӧʽ��________________��
(6)�����ѳ���SO2ֻ�����̿��е�MnO2��Ӧ������ͼʾ���̣���a m3(��״��)��SO2���������Ϊb%��β��ͨ�����SO2���ѳ���Ϊ89.6%�����յõ�MnO2������Ϊc kg�����ȥ��������ͭ����������ʱ�����������Ԫ���൱��MnO2________kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л��������ұ�(CB2760��2011)�涨���Ѿ���SO2���ʹ����Ϊ0.25 g��L��1��ij��ȤС����ͼ(a)װ��(�г�װ����)�ռ�ij���Ѿ��е�SO2�������京�����вⶨ��
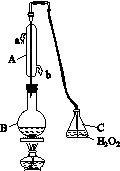
(a)
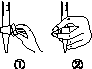 ��
��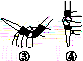
(b)
(1)����A��������__________________��ˮͨ��A�Ľ���Ϊ________��
(2)B�м���300.00 mL���Ѿƺ��������ᣬ����ʹSO2ȫ���ݳ�����C�е�H2O2��ȫ��Ӧ���仯ѧ����ʽΪ________________________��
(3)��ȥC�й�����H2O2��Ȼ����0.090 0 mol��L��1NaOH����Һ���еζ����ζ�ǰ������ʱ��Ӧѡ��ͼ(b)�е�________�����ζ��յ�ʱ��Һ��pH��8.8����ѡ���ָʾ��Ϊ________������50 mL�ζ��ܽ���ʵ�飬���ζ����е�Һ���ڿ̶ȡ�10�����������Һ������(�����)________(�٣�10 mL���ڣ�40 mL����<10 mL����>40 mL)��
(4)�ζ����յ�ʱ������NaOH��Һ25.00 mL�������Ѿ���SO2����Ϊ________ g��L��1��
(5)�òⶨ�����ʵ��ֵƫ�ߣ�����ԭ����������װ������Ľ���ʩ��________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������������������ֳ�����������д�ڿհ״�
��1����п������ϡ������ʱ��������ų���
��2��ʢ��Ũ������ձ����ڷ���һ��ʱ����������ӡ�
��3���ò�����պŨ�������ֽ��ʱ��ֽ��ڡ�
��4��ľ̿�����ȵ�Ũ������ʱ��������ų���
��5�������¿���������������������Ũ����
��6���ȵ�Ũ������ͭƬ�Ƚ�����Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��amL NO��bmL NO2��xmL O2�����ͬһ�Թ���,���Թܵ�����ˮ��,��������ˮ��ַ�Ӧ��,�Թ��ڵ�����ȫ����ʧ,��x��a��b�ĺ�����ϵʽx=f(a,b)Ϊ��(����)

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com