
Mg���仯������Է�������ת��(���ַ�Ӧ��������ˮ����ȥ)����֪X��Y��ZΪ��̬���ʣ�B������ΪҺ̬��D����ɫΪ��ɫ��C��G���ð���̲�����A��
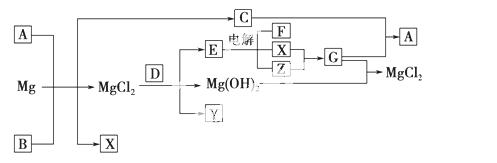
(1)д���������ʵĻ�ѧʽ��A________��Y________��
 (2)���õ���ʽ��ʾB���γɹ��̣�_________________________________
(2)���õ���ʽ��ʾB���γɹ��̣�_________________________________
_________________________________ ______��
______��
(3)���õ����ˮ��ƽ�����A��B ��Mg�D��C��X��MgCl2��ԭ��________
��Mg�D��C��X��MgCl2��ԭ��________
________________________________________________________________��
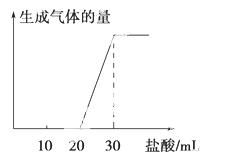
(4)��1 mol CO2ͨ��1 L����ΪF����Һ�У���ַ�Ӧ��������Һ����εμ����ᣬ�����������������ɵ� ����Ĺ�ϵ��ͼ��ʾ��
����Ĺ�ϵ��ͼ��ʾ�� ԭF��Һ�����ʵ���Ũ��Ϊ________ mol��L��1��
ԭF��Һ�����ʵ���Ũ��Ϊ________ mol��L��1��
 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±���A��B��C��D��E�����л�����й���Ϣ��
| A | ����ʹ������Ȼ�̼��Һ��ɫ�� �ڱ���ģ��Ϊ
|
| B | ����C��H����Ԫ����ɣ������ģ��Ϊ |
| C | ����C��H��O����Ԫ����ɣ�������Na��Ӧ�� ����E��Ӧ������Է�������Ϊ88���� |
| D | ����Է���������C��2��������C�������õ� |
| E | ����C��H��O����Ԫ����ɣ�����ˮ��Һ��ʹ��ɫʯ����Һ��� |
�ش��������⣺
A��E�У����������� ������ĸ����
A��ʹ������Ȼ�̼��Һ��ɫ��������Ӧ�Ļ�ѧ����ʽΪ ��
C����������D�Ļ�ѧ����ʽΪ ��
�л���B���е������� ������ţ���
����ɫ��ζ��Һ�壻���ж����۲�����ˮ�����ܶȱ�ˮ��
����ʹ����KMnO4��Һ����ˮ��ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڷ�Ӧ2NO2(g) N2O4(g)�ﵽƽ������¶Ȳ���ʱ��ʹc(NO2)/c(N2O4)��ֵ�����Բ�ȡ�� ��
N2O4(g)�ﵽƽ������¶Ȳ���ʱ��ʹc(NO2)/c(N2O4)��ֵ�����Բ�ȡ�� ��
A. ������䣬����NO2���� B. ������䣬����N2O4����
C. ʹ�������ԭ����2�� D. ʹ����������N2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ͳ������dz�������ɫ���������ڹ�ҵ������������Ҫ����;��
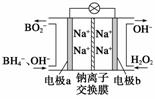 �žݱ��������⻯����NaBH4(BΪ��3��)��H2O2��ԭ�ϵ�ȼ�ϵ�أ�������ͨ�����ǵ�Դ���������ϲ���Pt��C���������ϲ���MnO2���乤��ԭ������ͼ��ʾ���õ�طŵ�ʱ�����ĵ缫��ӦʽΪ_________________________����MnO2���������ϣ���������Ϊ_____________________________��
�žݱ��������⻯����NaBH4(BΪ��3��)��H2O2��ԭ�ϵ�ȼ�ϵ�أ�������ͨ�����ǵ�Դ���������ϲ���Pt��C���������ϲ���MnO2���乤��ԭ������ͼ��ʾ���õ�طŵ�ʱ�����ĵ缫��ӦʽΪ_________________________����MnO2���������ϣ���������Ϊ_____________________________��
�ƻ�����䳣��Һ̬��(N2H4)Ϊȼ�ϣ�Һ̬��������Ϊ��ȼ������֪��
N2H4(l)��O2(g)===N2(g)��2H2O(g)����H����534 kJ��mol��1
 H2O2(l)===H2O(l)��
H2O2(l)===H2O(l)��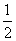 O2(g)����H����98 kJ��mol��1
O2(g)����H����98 kJ��mol��1
H2O(l)===H2O(g)����H����44 kJ��mol��1
��д��N2H4��Һ̬H2O2��Ӧ������̬ˮ���Ȼ�ѧ����ʽ___
__________________________________________��
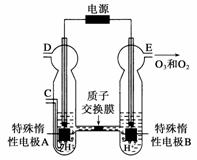
��O3���ɳ���������(ԭ������ͼ��ʾ)���ϡ�����Ƶá�
��ͼ������Ϊ________(�A����B��)��
����C��ͨ��O2����A���ĵ缫��ӦʽΪ____________��
����C����ͨ��O2��D��E���ֱ��ռ���15.68 L��6.72 L����(��״����)����E���ռ���������O2��O3�����֮��Ϊ__________(����O3�ķֽ�)��
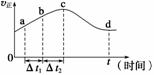 ������O3����������ȼú�����е�NOx��SO2�ѳ�Ч����������¯�����е�NOx 95%��������NO��ʽ���ڵģ��ɷ�����ӦNO(g)��O3(g)
������O3����������ȼú�����е�NOx��SO2�ѳ�Ч����������¯�����е�NOx 95%��������NO��ʽ���ڵģ��ɷ�����ӦNO(g)��O3(g) NO2(g)��O2(g)����һ�������£���NO��O3ͨ����Ⱥ����ܱ������з���������Ӧ������Ӧ������ʱ��
NO2(g)��O2(g)����һ�������£���NO��O3ͨ����Ⱥ����ܱ������з���������Ӧ������Ӧ������ʱ��
�仯��ʾ��ͼ����ͼ��ʾ����ͼ�ɵó�����ȷ˵����________��
a����Ӧ��c��ﵽƽ��״̬
b����Ӧ��Ũ�ȣ�b��С��c��
c���÷�ӦΪ���ȷ�Ӧ
d����t1����t2ʱ��NO��ת������a��b��С��b��c��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪A��B��C��D��E��F��G��H���Է�����ͼ��ʾ��ת������Ӧ�в�������������ȥ�����У�A��GΪͬһ����Ԫ�صĵ��ʣ�B��C��H��ͨ�������Ϊ���壬������C��һ���γ�����Ĵ�����Ⱦ�
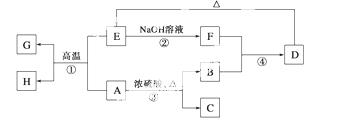
����գ�
(1)H��������________ __��
__��
(2)E��������;��__________��__________��
(3)��Ӧ�۵Ļ�ѧ����ʽ��__________________________________________ _____��
_____��
(4)��Ӧ�ܵ����ӷ���ʽ��_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����Һ������Fe2+��Fe3+��Ag+��Ba2+��Al3+��Ca2+��NH4+�����ӣ�����������ʵ�飨�����ᡢ�NH3ˮ��Br2ˮ���ǹ����ģ���
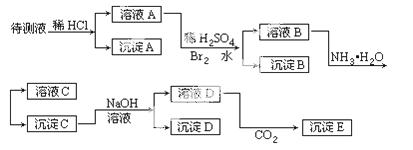
����ʵ������
��1���ж�����Һ������Ba2+��Ca2+���ӣ���д�����ɡ���_________��
��2��д����ѧʽ������A_________������D_________������E_________��
��3��д���������ӷ�Ӧ����ʽ����ˮ������ҺA�е�ij���ӣ�_________��
����C��������NaOH��Һ������һ�ֳ����ܽ⣺_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ʱ��ѡ�������������Ϊ���������ȷ���ǣ� ��
A. Cl2����ʯ�ң� B. SO2����ʯ�ң�
C. NH3����ˮ����ͭ�� D. HCl���Ȼ��ƻ����������ף�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
п���ϡ�����ᷴӦ��������п������狀�ˮ��������1 mol����пʱ������ԭ����������ʵ���Ϊ
A��2 mol B��1 mol C��0.5 mol D��0.25 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�ѧ������ȷ����
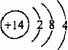
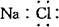 A���Ȼ��Ƶĵ���ʽ�� B����ϩ�Ľṹ��ʽ��C2H4
A���Ȼ��Ƶĵ���ʽ�� B����ϩ�Ľṹ��ʽ��C2H4
C�����ԭ�ӽṹʾ��ͼ�� D������ĵ��뷽��ʽ��Na2SO4=Na2����SO42��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com