

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
![]()
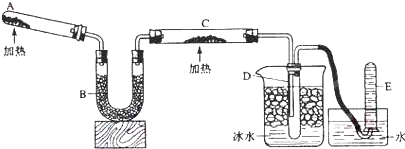
![]() ��֪�����������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ��
��֪�����������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ��
![]()
![]()

![]()
![]()
![]()
![]() �ش��������⣺
�ش��������⣺
![]()
![]() ��1��A����������_____________��_____________________________.
��1��A����������_____________��_____________________________.
![]()
![]() ������Ӧ�Ļ�ѧ����ʽ��_______________��_______________________;
������Ӧ�Ļ�ѧ����ʽ��_______________��_______________________;
![]()
![]() ��2��B����������_____��________����������_________��_______________:
��2��B����������_____��________����������_________��_______________:
![]()
![]() ��3��ʵ��ʱ��C�й۲쵽��������_____________��_________________��
��3��ʵ��ʱ��C�й۲쵽��������_____________��_________________��
![]()
![]() ������Ӧ�Ļ�ѧ����ʽ��_________________��____________________;
������Ӧ�Ļ�ѧ����ʽ��_________________��____________________;
![]()
![]() (4) ʵ��ʱ��D�й۲쵽��������________________��__________________��
(4) ʵ��ʱ��D�й۲쵽��������________________��__________________��
![]()
![]() D���ռ�����������_______��_______,��������ʵķ�����������_________��_____________.
D���ռ�����������_______��_______,��������ʵķ�����������_________��_____________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�����и߸�������ѧ��11�·��¿���ѧ�Ծ� ���ͣ�ʵ����
��12�֣�����֪�����������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ��

�ش��������⣺
��1��A�м���Ĺ�̬������� ��������Ӧ�Ļ�ѧ����ʽ�� ��
��2��B���������� ���������� ��
��3��ʵ��ʱ�ڹ۲쵽C�е������� ���������������������� ��������Ӧ�Ļ�ѧ����ʽ�� ��
��4��ʵ��ʱ��D�й۲쵽�������� ��D���ռ����������� ������������е�ijһ�����ʵķ����������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com