
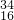 S2-Ī¢Į£ÖŠµÄÖŹ×ÓŹżŹĒ______£¬ÖŠ×ÓŹżŹĒ______£¬ŗĖĶāµē×ÓŹżŹĒ______£®±ź×¼×“æöĻĀ11.2LµÄ34SO2ĘųĢåµÄÖŹĮæŹĒ______£®
S2-Ī¢Į£ÖŠµÄÖŹ×ÓŹżŹĒ______£¬ÖŠ×ÓŹżŹĒ______£¬ŗĖĶāµē×ÓŹżŹĒ______£®±ź×¼×“æöĻĀ11.2LµÄ34SO2ĘųĢåµÄÖŹĮæŹĒ______£®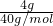 =0.1mol£¬¹ŹĒāŃõ»ÆÄĘČÜŅŗµÄÅضČĪŖ
=0.1mol£¬¹ŹĒāŃõ»ÆÄĘČÜŅŗµÄÅضČĪŖ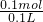 =1mol/L£¬ČÜŅŗŹĒ¾łŌČµÄ£¬Č”³ö10mLøĆČÜŅŗ£¬ÓėŌĒāŃõ»ÆÄĘČÜŅŗµÄÅضČĻąĶ¬ĪŖ1mol/L£¬øł¾ŻĻ”ŹĶ¶ØĀÉ£¬Ļ”ŹĶĒ°ŗóČÜÖŹĒāŃõ»ÆÄʵÄĪļÖŹµÄĮæ²»±ä£¬ĮīĻ”ŹĶŗóĒāŃõ»ÆÄĘČÜŅŗµÄÅضČĪŖc£¬Ōņ£ŗ10mL”Į1mol/L=100mL”Įc£¬½āµĆc=0.1mol/L£¬
=1mol/L£¬ČÜŅŗŹĒ¾łŌČµÄ£¬Č”³ö10mLøĆČÜŅŗ£¬ÓėŌĒāŃõ»ÆÄĘČÜŅŗµÄÅضČĻąĶ¬ĪŖ1mol/L£¬øł¾ŻĻ”ŹĶ¶ØĀÉ£¬Ļ”ŹĶĒ°ŗóČÜÖŹĒāŃõ»ÆÄʵÄĪļÖŹµÄĮæ²»±ä£¬ĮīĻ”ŹĶŗóĒāŃõ»ÆÄĘČÜŅŗµÄÅضČĪŖc£¬Ōņ£ŗ10mL”Į1mol/L=100mL”Įc£¬½āµĆc=0.1mol/L£¬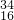 S2-Ī¢Į£ÖŠµÄÖŹ×ÓŹżĪŖ16£¬ÖŹĮæŹżĪŖ34£¬¹ŹÖŠ×ÓŹż=34-16=18£¬ŗĖĶāµē×ÓŹżĪŖ16+2=18£¬11.2LµÄ34SO2ĘųĢåµÄĪļÖŹµÄĮæĪŖ
S2-Ī¢Į£ÖŠµÄÖŹ×ÓŹżĪŖ16£¬ÖŹĮæŹżĪŖ34£¬¹ŹÖŠ×ÓŹż=34-16=18£¬ŗĖĶāµē×ÓŹżĪŖ16+2=18£¬11.2LµÄ34SO2ĘųĢåµÄĪļÖŹµÄĮæĪŖ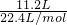 =0.5mol£¬¹ŹøƶžŃõ»ÆĮņµÄÖŹĮæĪŖ0.5mol”Į66g/mol=33g£¬
=0.5mol£¬¹ŹøƶžŃõ»ÆĮņµÄÖŹĮæĪŖ0.5mol”Į66g/mol=33g£¬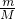 ¼ĘĖć4gNaOHµÄĪļÖŹµÄĮ棬øł¾Żc=
¼ĘĖć4gNaOHµÄĪļÖŹµÄĮ棬øł¾Żc= ¼ĘĖćĒāŃõ»ÆÄĘČÜŅŗµÄĪļÖŹµÄĮæÅØ¶Č£¬ČÜŅŗŹĒ¾łŌČµÄ£¬Č”³ö10mLøĆČÜŅŗ£¬ÓėŌĒāŃõ»ÆÄĘČÜŅŗµÄÅضČĻąĶ¬£¬øł¾ŻĻ”ŹĶ¶ØĀÉ£¬Ļ”ŹĶĒ°ŗóČÜÖŹĒāŃõ»ÆÄʵÄĪļÖŹµÄĮæ²»±ä£¬¾Ż“Ė¼ĘĖćĻ”ŹĶŗóµÄÅØ¶Č£»
¼ĘĖćĒāŃõ»ÆÄĘČÜŅŗµÄĪļÖŹµÄĮæÅØ¶Č£¬ČÜŅŗŹĒ¾łŌČµÄ£¬Č”³ö10mLøĆČÜŅŗ£¬ÓėŌĒāŃõ»ÆÄĘČÜŅŗµÄÅضČĻąĶ¬£¬øł¾ŻĻ”ŹĶ¶ØĀÉ£¬Ļ”ŹĶĒ°ŗóČÜÖŹĒāŃõ»ÆÄʵÄĪļÖŹµÄĮæ²»±ä£¬¾Ż“Ė¼ĘĖćĻ”ŹĶŗóµÄÅØ¶Č£»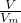 ¼ĘĖ涞Ńõ»ÆĮņµÄĪļÖŹµÄĮ棬ŌŁøł¾Żm=nM¼ĘĖ涞Ńõ»ÆĮņµÄÖŹĮ森
¼ĘĖ涞Ńõ»ÆĮņµÄĪļÖŹµÄĮ棬ŌŁøł¾Żm=nM¼ĘĖ涞Ńõ»ÆĮņµÄÖŹĮ森

 Ē§ĄļĀķ×ßĻņ¼ŁĘŚĘŚÄ©·ĀÕęŹŌ¾ķŗ®¼ŁĻµĮŠ“š°ø
Ē§ĄļĀķ×ßĻņ¼ŁĘŚĘŚÄ©·ĀÕęŹŌ¾ķŗ®¼ŁĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 ÉśĪļ֏׏Ō“ŹĒŅ»ÖÖĪŪČ¾Š”µÄæÉŌŁÉśÄÜŌ“£®ÉśĪļÖŹµÄÖ÷ŅŖ×Ŗ»ÆĶ¾¾¶¼°Ö÷ŅŖ²śĪļČēĶ¼£®
ÉśĪļ֏׏Ō“ŹĒŅ»ÖÖĪŪČ¾Š”µÄæÉŌŁÉśÄÜŌ“£®ÉśĪļÖŹµÄÖ÷ŅŖ×Ŗ»ÆĶ¾¾¶¼°Ö÷ŅŖ²śĪļČēĶ¼£®| ČŻĘ÷ | ¼× | ŅŅ | ±ū |
| ·“Ó¦ĪļĶ¶ČėĮæ | 1molCO”¢2molH2 | 1mol CH3OH | 2molCO”¢4molH2 |
| CH3OHµÄÅØ¶Č£Ømol/L£© | c1 | c2 | c3 |
| ·“Ó¦µÄÄÜĮæ±ä»Æ | ·Å³öQ1 kJ | ĪüŹÕQ2 kJ | ·Å³öQ3 kJ |
| Ę½ŗā³£Źż | K1 | K2 | K3 |
| ·“Ó¦Īļ×Ŗ»ÆĀŹ | ¦Į 1 | ¦Į 2 | ¦Į 3 |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢1molĘųĢåÖ»ÓŠŌŚ±ź×¼×“æöŹ±£¬ĘäĢå»ż²ÅÄÜĪŖ22.4L | B”¢ŌŚŅ»¶ØĪĀ¶ČŗĶŃ¹ĒæĻĀ£¬ĘųĢåĢå»żµÄ“óŠ”Ö÷ŅŖČ”¾öÓŚĘųĢå·Ö×ÓÖ®¼äµÄ¾ąĄė | C”¢Ķ¬ĪĀĶ¬Ń¹ĻĀ£¬N2ŗĶO2µÄ»ģŗĻĘųĢåÓėµČĢå»żµÄNOĘųĢå¾ßÓŠĻąĶ¬µÄŌ×ÓŹż | D”¢Na2O2Óė×ćĮæµÄĖ®·“Ӧɜ³É1 mol O2£¬Ėł×ŖŅʵĵē×ÓĪŖ4 mol |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
£Ø16·Ö£©ÉśĪļ֏׏Ō“ŹĒŅ»ÖÖĪŪČ¾Š”µÄæÉŌŁÉśÄÜŌ“”£ÉśĪļÖŹµÄÖ÷
£Ø1£©ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ ”” ”£
a£®ÉśĪļÖŹÄÜ£¬±¾ÖŹÉĻÄÜĮæĄ“Ō“ÓŚĢ«ŃōÄÜ
b£®ÓÉĻĖĪ¬ĖŲĖ®½ā»ńµĆµÄŅŅ“¼×÷Č¼ĮĻŹĒĄūÓĆĮĖÉśĪļÖŹÄÜ
c£®ÉśĪļÖŹĮŃ½ā»ńµĆµÄĘūÓĶ”¢²ńÓĶµČŹōÓŚ“æ¾»Īļ
d£®ÓÉÖ²Īļ½ÕøĖµČŃįŃõ·¢½Ķ»ńµĆµÄÕÓĘų£¬Ö÷ŅŖ³É·ÖŹĒ¼×Ķé
£Ø2£©ÓÉÉśĪļÖŹÄÜ»ńµĆµÄCOŗĶH2£¬æÉŅŌŗĻ³É¼×“¼ŗĶ¶ž¼×ĆŃ£ØCH3OCH3£©¼°Šķ¶ąĢžĄąĪļÖŹ”£µ±Į½Õß1”Ć1“߻Ʒ“Ó¦£¬ĘäŌ×ÓĄūÓĆĀŹ“ļ100%£¬ŗĻ³ÉµÄĪļÖŹæÉÄÜŹĒ ”£
a£®ĘūÓĶ b£®¼×“¼ c£®¼×Č© d£®ŅŅĖį
£Ø3£©¼×“¼ŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬¹¤ŅµÉĻŗĻ³É¼×“¼µÄ·“Ó¦£ŗ
CO(g)£«2H2(g)CH3OH(g) ”÷H= -90.8kJ”¤mol-1”£
ČōŌŚĪĀ¶Č”¢ČŻ»żĻąĶ¬µÄ3øöĆܱÕČŻĘ÷ÖŠ£¬°“²»Ķ¬·½Ź½Ķ¶Čė·“Ó¦Īļ£¬±£³ÖŗćĪĀ”¢ŗćČŻ£¬²āµĆ·“Ó¦“ļµ½Ę½ŗāŹ±µÄÓŠ¹ŲŹż¾ŻČēĻĀ£ŗ
| ČŻĘ÷ | ¼× | ŅŅ | ±ū |
| ·“Ó¦ĪļĶ¶ČėĮæ | 1molCO ”¢2molH2 | 1mol CH3OH | 2molCO”¢4molH2 |
| CH3OHµÄÅØ¶Č£Ømol/L£© | c1 | c2 | c3 |
| ·“Ó¦µÄÄÜĮæ±ä»Æ | ·Å³öQ1 kJ | ĪüŹÕQ2 kJ | ·Å³öQ3 kJ |
| Ę½ŗā³£Źż | K1 | K2 | K3 |
| ·“Ó¦Īļ×Ŗ»ÆĀŹ | ¦Į 1 | ¦Į 2 | ¦Į 3 |
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ ”£
a. c1 = c2 b. 2Q1 £½ Q3 c. K1=K3 d. ¦Į2+ ¦Į3£¼ 100%
£Ø4£©ŌŚŅ»¶ØĪĀ¶ČŗĶŃ¹ĒæĻĀ£¬COŗĶH2“ß»ÆŗĻ³É¶ž¼×Ćѵķ“Ó¦ĪŖ£ŗ
3H2£Øg£©+3CO£Øg£© CH3OCH3£Øg£©+CO2£Øg£©
¢ŁČōŅ»Ģå»żæɱäµÄĆܱÕČŻĘ÷ÖŠ³äČė3 mol H2”¢3 mol CO”¢1 mol CH3OCH3”¢1 mol CO2£¬¾Ņ»¶ØŹ±¼ä“ļµ½Ę½ŗā£¬²¢²āµĆĘ½ŗāŹ±»ģŗĻĘųĢåĆܶȏĒĶ¬ĪĀĶ¬Ń¹ĻĀĘšŹ¼Ź±µÄ1.6±¶”£Ōņ£ŗ¢Ł·“Ó¦æŖŹ¼Ź±Õż”¢Äę·“Ó¦ĖŁĀŹµÄ“󊔣ŗv(Õż£©____v(Äę)£ØĢī”° >”±”¢”° < ”±»ņ”°=”±£©£¬ĄķÓÉŹĒ
”£Ę½ŗāŹ±n(CH3OCH3)= mol”£
¢ŚÓŅĶ¼ĪŖĀĢÉ«µēŌ“”°Ö±½Ó¶ž¼×ĆŃČ¼ĮĻµē³Ų”±µÄ¹¤×÷ŌĄķŹ¾ŅāĶ¼”£
bµē¼«ŹĒ ¼«£»aµē¼«µÄ·“Ó¦Ź½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011½ģÕć½Ź”ŗ¼ÖŻŹŠøßČżµŚ¶ž“ĪÖŹ¼ģ£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
(15·Ö)ÉśĪļ֏׏Ō“ŹĒŅ»ÖÖĪŪČ¾Š”µÄæÉŌŁÉśÄÜŌ“”£ÉśĪļÖŹµÄÖ÷ŅŖ×Ŗ»ÆĶ¾¾¶¼°Ö÷ŅŖ²śĪļČēĻĀĶ¼”£
£Ø1£©ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ ”” ”£
a£®ÉśĪļÖŹÄÜ£¬±¾ÖŹÉĻÄÜĮæĄ“Ō“ÓŚĢ«ŃōÄÜ
b£®ÓÉĻĖĪ¬ĖŲĖ®½ā»ńµĆµÄŅŅ“¼×÷Č¼ĮĻŹĒĄūÓĆĮĖÉśĪļÖŹÄÜ
c£®ÉśĪļÖŹĮŃ½ā»ńµĆµÄĘūÓĶ”¢²ńÓĶµČŹōÓŚ“æ¾»Īļ
d£®ÓÉÖ²Īļ½ÕøĖµČŃįŃõ·¢½Ķ»ńµĆµÄÕÓĘų£¬Ö÷ŅŖ³É·ÖŹĒ¼×Ķé
£Ø2£©ÓÉÉśĪļÖŹÄÜ»ńµĆµÄCOŗĶH2£¬æÉŅŌŗĻ³É¼×“¼ŗĶ¶ž¼×ĆŃ£ØCH3OCH3£©¼°Šķ¶ąĢžĄąĪļÖŹ”£µ±Į½Õß1”Ć1“߻Ʒ“Ó¦£¬ĘäŌ×ÓĄūÓĆĀŹ“ļ100%£¬ŗĻ³ÉµÄĪļÖŹæÉÄÜŹĒ ”£
a£®ĘūÓĶ b£®¼×“¼ c£®¼×Č© d£®ŅŅĖį
£Ø3£©¼×“¼ŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬¹¤ŅµÉĻŗĻ³É¼×“¼µÄ·“Ó¦£ŗ
CO(g)£«2H2(g) CH3OH(g) ”÷H= -90.8kJ”¤mol-1”£
CH3OH(g) ”÷H= -90.8kJ”¤mol-1”£
ČōŌŚĪĀ¶Č”¢ČŻ»żĻąĶ¬µÄ3øöĆܱÕČŻĘ÷ÖŠ£¬°“²»Ķ¬·½Ź½Ķ¶Čė·“Ó¦Īļ£¬±£³ÖŗćĪĀ”¢ŗćČŻ£¬²āµĆ·“Ó¦“ļµ½Ę½ŗāŹ±µÄÓŠ¹ŲŹż¾ŻČēĻĀ£ŗ
| ČŻĘ÷ | ¼× | ŅŅ | ±ū |
| ·“Ó¦ĪļĶ¶ČėĮæ | 1molCO ”¢2molH2 | 1mol CH3OH | 2molCO”¢4molH2 |
| CH3OHµÄÅØ¶Č£Ømol/L£© | c1 | c2 | c3 |
| ·“Ó¦µÄÄÜĮæ±ä»Æ | ·Å³öQ1 kJ | ĪüŹÕQ2 kJ | ·Å³öQ3 kJ |
| Ę½ŗā³£Źż | K1 | K2 | K3 |
| ·“Ó¦Īļ×Ŗ»ÆĀŹ | ¦Į1 | ¦Į 2 | ¦Į3 |
 CH3OCH3£Øg£©+CO2£Øg£©
CH3OCH3£Øg£©+CO2£Øg£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com