
 ����̼̼˫�������Ա�һ����ʹ ��ɫ��
����̼̼˫�������Ա�һ����ʹ ��ɫ�� ��̼ԭ��֮��Ľ� ��6��̼ԭ�Ӻ�6����ԭ�Ӷ���ͬһ �ϡ�
��̼ԭ��֮��Ľ� ��6��̼ԭ�Ӻ�6����ԭ�Ӷ���ͬһ �ϡ� һ���̼̼����˫����Ļ�״�ṹ��
һ���̼̼����˫����Ļ�״�ṹ��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
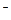 ��OH
��OH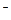 ��SO
��SO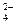 �������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ������ǿ�����CO2��Ӱ�죩��
�������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ������ǿ�����CO2��Ӱ�죩��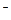
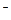 ��
�� L-1H2SO4��0.01mol
L-1H2SO4��0.01mol L-1KMnO4����ɫʯ����Һ����ÿ��2�֣�
L-1KMnO4����ɫʯ����Һ����ÿ��2�֣�| ʵ�鲽�� | Ԥ������ͽ��� |
����1��ȡ��������Һ���Թ��У��μ�3 moL L-1 H2SO4����Һ�����ԣ�Ȼ��������Һ������A��B�Թ��У� L-1 H2SO4����Һ�����ԣ�Ȼ��������Һ������A��B�Թ��У� | |
| ����2�� | |
| ����3�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ |
| B���ζ��յ����ʱ���Ӷ��� |
| C��������ˮϴ����ʽ�ζ��ܺ�װ���������еζ� |
| D����ƿˮϴ��δ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������Ũ��ˮ��Fe���������屽 |
| B����������NaOH��Һ���ȣ�ˮ������AgNO3��Һ����Cl- |
| C��CH4��Cl2�����Ƶô�����CH3Cl |
| D����ϩͨ�뺬Br2��CCl4��Һ�Ƶ�CH2BrCH2Br |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ����֤����ȫȼ�պ����ֻ��H2O��CO2 |
| B���ⶨ����������������������ȫȼ�պ�����CO2��H2O������ |
| C���ⶨ��ȫȼ��ʱ�����л��������ɵ�CO2��H2O�����ʵ���֮�� |
| D��ֻ�вⶨ��ȼ�ղ�����H2O��CO2���ʵ����ı�ֵ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����




 ��IBr ��
��IBr �� ��IBr��KI��I2��KBr ��I2��2S2O32����2I����S4O62��
��IBr��KI��I2��KBr ��I2��2S2O32����2I����S4O62��


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com