
�����ʵ���Ũ�Ⱦ�Ϊ0.1mol��L��1��HA��BOH��Һ�������ϣ�����˵���������
| A����HAΪ���ᣬBOHΪ�������C(H��)+C(B��)===C(OH��)+ C(A��) |
| B����HAΪǿ�ᣬBOHΪ�������C(A��)��C(B��) ��C(H��) ��C(OH��) |
| C����HAΪ���ᣬBOHΪǿ�����C(B��) ��C(A��) ��C(OH��) ��C(H��) |
| D����HAΪǿ�ᣬBOHΪǿ�����C(H��)= C(A��)= C(B��)= C(OH��)=0.1mol��L��1 |
 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
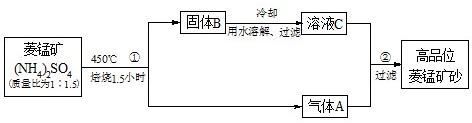
| ||
| c(Ba2+) |
| c(Mn2+) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��V1��V2=3��2 | B��V1��V2=2��1 | C��V1��V2=1��2 | D��V1��V2=2��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����ʵ���Ũ�Ⱦ�Ϊ0.1mol��L��1��HA��BOH��Һ�������ϣ�����˵���������
A����HAΪ���ᣬBOHΪ�������C(H��)+C(B��)===C(OH��)+ C(A��)
B����HAΪǿ�ᣬBOHΪ�������C(A��)��C(B��) ��C(H��) ��C(OH��)
C����HAΪ���ᣬBOHΪǿ�����C(B��) ��C(A��) ��C(OH��) ��C(H��)
D����HAΪǿ�ᣬBOHΪǿ�����C(H��)= C(A��)= C(B��)= C(OH��)=0.1mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com