
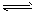 H2+I2
H2+I2
 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ͨ�� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��
��SO2��2H2O��I2===H2SO4��2HI
��2HIH2��I2
��2H2SO4===2SO2��O2��2H2O
(1)����������Ӧ�������ж���ȷ����________(����)��
a����Ӧ�����ڳ����½���
b����Ӧ����SO2�����Ա�HIǿ
c��ѭ���������貹��H2O
d��ѭ�����̲���1 mol O2��ͬʱ����1 mol H2
(2)һ���¶��£���1 L�ܱ������м���1 mol HI(g)��������Ӧ��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��
��0��2 min�ڵ�ƽ����Ӧ����v(HI)�� �����¶��£�H2(g)��I2(g)
2HI(g)��ƽ�ⳣ��K�� ��
����ͬ�¶��£�����ʼ����HI(g)�����ʵ�����ԭ����2������ ��ԭ����2����
a��ƽ�ⳣ��
b��HI��ƽ��Ũ��
c���ﵽƽ���ʱ��
d��ƽ��ʱH2���������
(3)ʵ������Zn��ϡ������ȡH2����Ӧʱ��Һ��ˮ�ĵ���ƽ�� �ƶ�(��������ҡ�����)�����������������Լ��е� ������H2�����ʽ�����
a��NaNO3 b��CuSO4
c��Na2SO4 d��NaHSO3
(4)��H2Ϊȼ�Ͽ���������ȼ�ϵ�ء�
��֪2H2(g)��O2(g)===2H2O(l)����H����572 kJ��mol��1,ij����ȼ�ϵ���ͷ�228.8 kJ����ʱ������1 molҺ̬ˮ���õ�ص�����ת����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ӱ�ʡ������ѧ�ڵ������¿���ѧ�Ծ��������棩 ���ͣ������
��7�֣���ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��
��. SO2��2H2O��I2===H2SO4��2HI
��. 2HI H2��I2
H2��I2
��. 2H2SO4===2SO2��O2��2H2O
(1) ����������Ӧ�������ж���ȷ���� _______________
a����Ӧ�����ڳ����½���
b����Ӧ����SO2�����Ա�HIǿ
c��ѭ���������貹��H2O
d��ѭ�����̲���1 mol O2��ͬʱ����1 mol H2
(2) һ���¶��£���1 L�ܱ������м���1 mol HI(g)��������Ӧ��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��
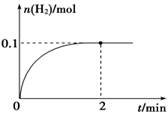
�� 0��2 min�ڵ�ƽ����Ӧ����v(HI)�� ________________
�� ���¶��£�H2(g)��I2(g)  2HI(g)��ƽ�ⳣ��K�� __________________
2HI(g)��ƽ�ⳣ��K�� __________________
�� ��ͬ�¶��£�����ʼ����HI(g)�����ʵ�����ԭ����2������ _____________��ԭ����2����
a��ƽ�ⳣ�� b��HI��ƽ��Ũ��
c���ﵽƽ���ʱ�� d��ƽ��ʱH2���������
(3) ʵ������Zn��ϡ������ȡH2����Ӧʱ���������������Լ��е�____________����H2�����ʽ�����
a��NaNO3 b��CuSO4 c��Na2SO4 d��NaHSO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡΫ���и߶�������ҵ��ѧ�������Ծ� ���ͣ������
��ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��
��SO2��2H2O��I2===H2SO4��2HI
��2HI H2��I2
H2��I2
��2H2SO4===2SO2��O2��2H2O
(1)����������Ӧ�������ж���ȷ����________(����)��
a����Ӧ�����ڳ����½���
b����Ӧ����SO2�����Ա�HIǿ
c��ѭ���������貹��H2O
d��ѭ�����̲���1 mol O2��ͬʱ����1 mol H2
(2)һ���¶��£���1 L�ܱ������м���1 mol HI(g)��������Ӧ��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��

��0��2 min�ڵ�ƽ����Ӧ����v(HI)��
�����¶��£�H2(g)��I2(g) 
2HI(g)��ƽ�ⳣ��K�� ��
����ͬ�¶��£�����ʼ����HI(g)�����ʵ�����ԭ����2������ ��ԭ����2����
a��ƽ�ⳣ��
b��HI��ƽ��Ũ��
c���ﵽƽ���ʱ��
d��ƽ��ʱH2���������
(3)ʵ������Zn��ϡ������ȡH2����Ӧʱ��Һ��ˮ�ĵ���ƽ�� �ƶ�(��������ҡ�����)�����������������Լ��е� ������H2�����ʽ�����
a��NaNO3 b��CuSO4
c��Na2SO4 d��NaHSO3
(4)��H2Ϊȼ�Ͽ���������ȼ�ϵ�ء�
��֪2H2(g)��O2(g)===2H2O(l)����H����572 kJ��mol��1,ij����ȼ�ϵ���ͷ�228.8 kJ����ʱ������1 molҺ̬ˮ���õ�ص�����ת����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010����ͨ�ߵ�ѧУ����ȫ��ͳһ�������ۻ�ѧ���֣�ɽ������ ���ͣ������
����ѭ���ֽ�ˮ������Ҫ�漰���·�Ӧ
I SO2��2H2O��I2=H2SO4��2HI
II
2HI H2��I2
H2��I2
III 2H2SO4=2SO2��O2��2H2O
��1������������Ӧ�������ж���ȷ����__________
a.��ӦIII���ڳ����½��� b.��ӦI��SO2�����Ա�HIǿ
c.ѭ���������貹��H2O d.ѭ�������в���1mol O2��ͬʱ����1 molH2
��2��һ���¶��£���1L�ܱ������м���1 molHI��g����������ӦII��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��

0��2min�Ѽ�������ƽ����Ӧ����v(HI)=_____________�����¶��£�H2(g)��I2(g) 2HI(g)��ƽ�ⳣ��K=__________��
2HI(g)��ƽ�ⳣ��K=__________��
��ͬ�¶��£�����ʼ����2HI(g)�����ʵ�����ԭ����2������_____��ԭ����2����
a.ƽ�ⳣ�� b.HI��ƽ��Ũ�� c.�ﵽƽ���ʱ�� d.ƽ��ʱH2���������
��3��ʵ������Zn��ϡ������H2����Ӧʱ����Һ��ˮ�ĵ���ƽ��________�ƶ�����������ҡ������������������������Լ��е�__________������H2�����ʽ�����
A�� NaNO3 B.CuSO4 C .Na2SO4 D .NaHSO3
(4)��H2Ϊȼ�Ͽ���������ȼ�ϵ�ء�
��֪ 2H2��g��+O2��g��=2H2O(1) ��H=-572kj��mol-1
ij����ȼ�ϵ���ͷ�228.8kj����ʱ������1molҺ̬ˮ���õ�ص�����ת����Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com