
(8��)(1)2003��3���ձ��������Ͽ�ѧ����ʵ����һ���о�С�鷢���������ᾧˮ�ľ�����5K�³��ֳ����ԡ��ݱ������þ���Ļ�ѧʽΪ Na0.35CoO2• 1.3H2O���Լ��㣺�þ�������ԭ������ԭ�ӵ����ʵ���֮����___��_____��1mol�þ����к��е���ԭ����Ŀ��____��_______��
(2)���ơ��⡢����������Ԫ�أ������е�һ�ֻ���Ԫ�ؿ�����ɶ������ʣ�д����������Ҫ��Ļ�ѧ����ʽ��
������������ͼӦ������������������������������������������
�ڼ�����������ᷴӦ�������������� ����
 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �������� | ���� | ���� | ���� | ���� | ��� | ��� | ��� |
| �е�/�� | -196 | -183 | -269 | -264 | -186 | -153 | -108 |
| ԭ�� | ��Ȼ�� | ���� | ú |
| ���Ͷ�ʷ��� | 1.0 | 1.5 | 2.0 |
| ��������/J?t-1 | 28��109 | 38��109 | 48��109 |
| ||
| ���� |
| ||
| ���� |
| NH3����% ѹǿ/MPa �¶�/�� |
0.1 | 10 | 20 | 30 | 60 | 100 |
| 200 | 15.3 | 81.5 | 86.4 | 89.9 | 95.4 | 98.8 |
| 300 | 2.2 | 52.0 | 64.2 | 71.0 | 84.2 | 92.6 |
| 400 | 0.4 | 25.1 | 38.2 | 47.0 | 65.2 | 79.8 |
| 500 | 0.1 | 10.6 | 19.1 | 26.4 | 42.2 | 57.5 |
| 600 | 0.05 | 4.5 | 9.1 | 13.8 | 23.1 | 31.4 |
 2NH3�ġ�H
2NH3�ġ�H| c2(NH3) |
| c(N2)?c3(H2) |
| c2(NH3) |
| c(N2)?c3(H2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����8�֣�
0.80gCuSO4��5H2O��Ʒ������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ�����ͼ��ʾ��
��ش��������⣺
��1����ȷ��200��ʱ�������ʵĻ�ѧʽ______________��Ҫ��д���ƶϹ��̣�
��2��ȡ270��������Ʒ����570�����յõ�����Ҫ�����Ǻ�ɫ��ĩ��һ�����������壬�÷�Ӧ�Ļ�ѧ����ʽΪ______________���Ѹú�ɫ��ĩ�ܽ���ϡ�����У���Ũ������ȴ���о����������þ���Ļ�ѧʽΪ_________������ڵ�����¶���_____________��
��3������������������ˮ��Ӧ����һ�ֻ�����û������Ũ��Һ��Cu�ڼ���ʱ������Ӧ�Ļ�ѧ����ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��) ��֪�л���A����Է�������������200��ȡ1.48 g A��ȫȼ�պ�ȼ�ղ���ͨ����ʯ�ң���ʯ�ҵ���������2.12g������ȼ�ղ���ͨ��Ũ���ᣬŨ�������������0.36 g��ȡ1.48 g A�������Ʒ�Ӧ�����ɵ������ڱ�״���µ����Ϊ0.336L��
(1) 1.48 g A��ȫȼ�����ɵ�CO2�����ʵ���Ϊ________mol��
(2) A�ķ���ʽΪ_______________��
(3) A��ʹ��ɫʯ����Һ��죬��A���������������ֻ��һ����ԭ�ӣ���A�Ľṹ��ʽΪ_____________��
(4) ��a g A��ȫȼ�պ�IJ���ȫ��ͨ��������Na2O2���������գ��������������
____________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�人����У����10��������ѧ�Ծ��������棩 ���ͣ������
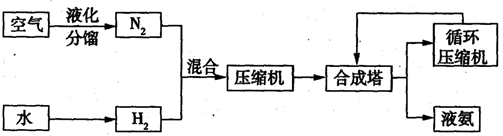
(8��)�й�������ŵ����2020�꣬��λGDP������̼�ŷű�2005���½�40%~50%��
��1����Ч����̼�����ֶ�֮һ�ǽ��ܣ��������ⷽ������ܵ���
A�����ˮ���⣺2H2O 2H2����O2��
2H2����O2��
B������ʹˮ�ֽ����⣺2H2O 2H2����O2��
2H2����O2��
C��̫������ֽ�ˮ���⣺2H2O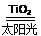 2H2����O2��
2H2����O2��
D����Ȼ�����⣺CH4��H2O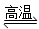 CO��3H2
CO��3H2
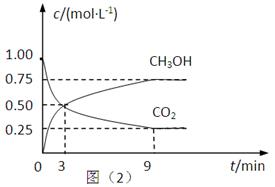
��2��CO2��ת�����л���ʵ��̼ѭ���������Ϊ1L���ܱ������У�����1mol CO2��3mol H2��һ�������·�Ӧ��CO2(g)+3H2(g) CH3OH(g)+H2O(g) ��H=��49.0kJ��mol��1�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ��
CH3OH(g)+H2O(g) ��H=��49.0kJ��mol��1�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ��
�ٴ�3 min��9 min��v(H2)=________mol��L��1��min��1��
����˵��������Ӧ�ﵽƽ��״̬����____________�����ţ���
A����Ӧ��CO2��CH3OH�����ʵ���Ũ��֮��Ϊ1��1����ͼ�н���㣩
B�����������ܶȲ���ʱ��ı仯���仯
C����λʱ��������3mol H2��ͬʱ����1mol H2O
D��CO2����������ڻ�������б��ֲ���
��3����ҵ�ϣ�CH3OHҲ����CO��H2�ϳɡ��ο��ϳɷ�ӦCO(g)+2H2(g) CH3OH(g)��ƽ�ⳣ����
CH3OH(g)��ƽ�ⳣ����
|
�¶�/�� |
0 |
100 |
200 |
300 |
400 |
|
ƽ�ⳣ�� |
667 |
13 |
1.9��10-2 |
2.4��10-4 |
1��10-5 |
����˵����ȷ����_____��
A���÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ
B���÷�Ӧ�ڵ����²����Է����У������¿��Է����У�˵���÷�Ӧ��S��0
C����T��ʱ��1L�ܱ������У�Ͷ��0.1mol CO��0.2 mol H2���ﵽƽ��ʱ��COת����Ϊ50%�����ʱ��ƽ�ⳣ��Ϊ100
D����ҵ�ϲ����Ըߵ�ѹǿ(5Mpa)��250�棬����Ϊ�������£�ԭ����ת�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ӱ�ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
(8��)�±���ʾ�ϳɰ���Ӧ��N2+3H2  2NH3���ڲ�ͬ�����´ﵽƽ��ʱ������а��ĺ���[��ʼʱv��N2����v��H2��==1��3]��
2NH3���ڲ�ͬ�����´ﵽƽ��ʱ������а��ĺ���[��ʼʱv��N2����v��H2��==1��3]��
|
�¶ȣ��棩 |
0.1 |
10 |
30 |
60 |
100 |
|
200 |
0.153 |
0.815 |
0.899 |
0.954 |
0.988 |
|
300 |
0.022 |
0.520 |
0.710 |
0.842 |
0.926 |
|
400 |
0.004 |
0.251 |
0.470 |
0.652 |
0.798 |
�����ϱ�����,�ش��������⣺
��1��200�桢100MPaʱ��ƽ�������а��ĺ����Ѵ�0.988�������������ѹǿ
����ܡ����ܡ���ʹƽ�������а��ĺ�������1�������ǣ�
��
��2����ʹƽ�������а��ĺ���������ɲ�ȡ�Ĵ�ʩ�У� ��
��3�� ��ʹƽ�������а��ĺ���Ϊ0.710����ѡ��ķ�Ӧ����ӦΪ��
��ʹƽ�������а��ĺ���Ϊ0.710����ѡ��ķ�Ӧ����ӦΪ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com