
ĄūÓĆÖĘČ”ĮņĖį²śÉśµÄæóŌü(Ö÷ŅŖ³É·ÖĪŖFe2O3”¢Fe3O4”¢FeO”¢Al2O3¼°SiO2µČ)Öʱøøß“æŃõ»ÆĢś(¦Į£Fe2O3)µÄ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
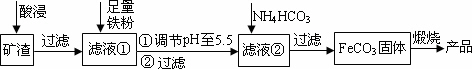
(1)”°Ėį½ž”±¹ż³ĢÖŠFe3O4·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ________£®ĪŖĢįøß”°Ėį½ž”±ÖŠĢśŌŖĖŲµÄ½ž³öĀŹ£¬³żĮĖ²ÉÓĆŗĻŹŹµÄŅŗ¹Ģ±ČŗĶŃ»·½žČ”Ķā£¬ŹŹŅĖµÄĢõ¼ž»¹ÓŠ________”¢
________£®(ČĪ¾ŁĮ½Ąż)
(2)µ÷½ŚpHÖĮ5.5µÄÄæµÄŹĒ________£®
(3)ĀĖŅŗ¢ŚÖŠ¼ÓČėNH4HCO3Ź±ŠčŅŖæŲÖĘ·“Ó¦ĪĀ¶Č²»Äܹżøߣ¬ŌŅņŹĒ________£®(“šŅ»µć¼“æÉ)
(4)ŌŚæÕĘųÖŠģŃÉÕFeCO3Öʱøøß“æŃõ»ÆĢśµÄ»Æѧ·½³ĢŹ½ĪŖ________£®
 Ó¢²Åµć½ņĻµĮŠ“š°ø
Ó¢²Åµć½ņĻµĮŠ“š°ø ŗģ¹ū×ÓČż¼¶²āŹŌ¾ķĻµĮŠ“š°ø
ŗģ¹ū×ÓČż¼¶²āŹŌ¾ķĻµĮŠ“š°ø æĪĢĆĮ·¼Ó²āĻµĮŠ“š°ø
æĪĢĆĮ·¼Ó²āĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ģŃÉÕ»¹Ō |
| Ė®½žČ” |
| ¹żĀĖ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
Ć¾ŌŚæÕĘųÖŠČ¼ÉÕ³żÉś³ÉMgOĶā£¬»¹æÉÄÜÉś³ÉMg3N2 ”£Ä³Š£»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§ĄūÓĆĆ¾ŌŚæÕĘųÖŠČ¼ÉÕŗóµÄ¹ĢĢå£Ø²»ŗ¬µ„ÖŹ£©½ųŠŠŹµŃ飬Ģ½¾æĘä×é³É”£
£Ø1£©¼××éĶ¬Ń§Č”Ņ»¶ØĮæČ¼ÉÕŗóµÄ¹ĢĢåĶ¶ČėĖ®ÖŠ£¬µĆµ½ĮĖŅ»ÖÖÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶ µÄĘųĢ壬øĆĘųĢåµÄ»ÆѧŹ½ĪŖ £¬ĖµĆ÷¹ĢĢåÖŠŗ¬ÓŠMg3N2£¬Éś³ÉøĆĘųĢåµÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø2£©ŅŅ×éĶ¬Ń§ĪŖ²ā¶ØMg3N2ŗ¬Į棬ÓĆĶ¼Ź¾×°ÖĆ½ųŠŠŹµŃ飬³ä·Ö·“Ó¦ŗóŌŁ¼ÓČČA”£ĘäÖŠÅØĮņĖįµÄ×÷ÓĆŹĒ £¬¶ŌA¼ÓČȵÄÄæµÄŹĒ ”£ŅŃÖŖ¼ÓČėµÄ¹ĢĢåÖŹĮæĪŖ4.0g£¬×īÖÕC×°ÖĆŌöÖŲag£¬Ōņ¹ĢĢåÖŠŗ¬Mg3N2 g£ØÓĆŗ¬aµÄŹ½×Ó±ķŹ¾£©”£

£Ø3£©±ū×éÓŠµÄĶ¬Ń§ČĻĪŖŅŅ×éĶ¬Ń§µÄ²ā¶Ø½į¹ūĘ«øߣ¬ĄķÓÉŹĒ
ӣ
ÓŠµÄĶ¬Ń§ČĻĪŖŅŅ×éĶ¬Ń§µÄ²ā¶Ø½į¹ūĘ«µĶ£¬ĄķÓÉŹĒ
ӣ
±ū×éĶ¬Ń§½ųŠŠĮĖøĽų£¬ĖūĆĒ½«ŅŅ×éĶ¬Ń§ŹµŃéÖŠµĆµ½µÄÄŃČܹĢĢå½ųŠŠ¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ£¬²¢
×ĘÉÕ¹ĢĢåÖĮŗćÖŲ£¬²āµĆĘäÖŹĮæĪŖ4.08g”£ÉĻŹö¹ż³ĢÖŠ£¬Ļ“µÓ³ĮµķµÄ²Ł×÷ŹĒ
ӣ
Ć¾ŌŚæÕĘųÖŠČ¼ÉÕŗóÉś³ÉµÄ¹ĢĢåÖŠMg3N2µÄÖŹĮæ·ÖŹżĪŖ ”£
ÓŠŅ»ÖÖÓĆļ§ŃĪÓėŗ¬Ć¾æóŹÆ»ģŗĻģŃÉÕÖĘČ”Ńõ»ÆĆ¾µÄ·½·Ø£¬½ā¾öĮĖĻÖÓŠ·½·Ø“ęŌŚµÄŌĮĻ³É ±¾øß”¢ĻīÄæĶ¶×Ź“ó”¢ÄÜŗÄøß”¢ø±²śĘ·²»ŗĆÓƵČĪŹĢā£¬ĘäŌĄķŹĒ½«ŗ¬Ć¾æóŹÆ·Ū£Øŗ¬Ńõ»ÆĆ¾£©Óė ļ§ŃĪ»ģŗĻ,¾¹żģŃÉÕ”¢Ė®ČÜ”¢¹żĀĖ£¬µĆµ½“ÖĆ¾ŃĪČÜŅŗ£¬²¢»ŲŹÕģŃÉÕ²śÉśµÄ°±”£Š“³öÓĆ¹ĢĢå£ØNH4£©2SO4Óėŗ¬Ć¾æóŹÆ·ŪģŃÉյĻÆѧ·“Ó¦·½³ĢŹ½
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011½ģ°²»ÕŹ”ĆūŠ£øßČżÉĻѧʌµŚŅ»“ĪĮŖæ¼£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗŹµŃéĢā
Ć¾ŌŚæÕĘųÖŠČ¼ÉÕ³żÉś³ÉMgOĶā£¬»¹æÉÄÜÉś³ÉMg3N2”£Ä³Š£»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§ĄūÓĆĆ¾ŌŚæÕĘųÖŠČ¼ÉÕŗóµÄ¹ĢĢå£Ø²»ŗ¬µ„ÖŹ£©½ųŠŠŹµŃ飬Ģ½¾æĘä×é³É”£
£Ø1£©¼××éĶ¬Ń§Č”Ņ»¶ØĮæČ¼ÉÕŗóµÄ¹ĢĢåĶ¶ČėĖ®ÖŠ£¬µĆµ½ĮĖŅ»ÖÖÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶ µÄĘųĢ壬øĆĘųĢåµÄ»ÆѧŹ½ĪŖ £¬ĖµĆ÷¹ĢĢåÖŠŗ¬ÓŠMg3N2£¬Éś³ÉøĆĘųĢåµÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø2£©ŅŅ×éĶ¬Ń§ĪŖ²ā¶ØMg3N2ŗ¬Į棬ÓĆĶ¼Ź¾×°ÖĆ½ųŠŠŹµŃ飬³ä·Ö·“Ó¦ŗóŌŁ¼ÓČČA”£ĘäÖŠÅØĮņĖįµÄ×÷ÓĆŹĒ £¬¶ŌA¼ÓČȵÄÄæµÄŹĒ ”£ŅŃÖŖ¼ÓČėµÄ¹ĢĢåÖŹĮæĪŖ4.0g£¬×īÖÕC×°ÖĆŌöÖŲag£¬Ōņ¹ĢĢåÖŠŗ¬Mg3N2 g£ØÓĆŗ¬aµÄŹ½×Ó±ķŹ¾£©”£
£Ø3£©±ū×éÓŠµÄĶ¬Ń§ČĻĪŖŅŅ×éĶ¬Ń§µÄ²ā¶Ø½į¹ūĘ«øߣ¬ĄķÓÉŹĒ
ӣ
ÓŠµÄĶ¬Ń§ČĻĪŖŅŅ×éĶ¬Ń§µÄ²ā¶Ø½į¹ūĘ«µĶ£¬ĄķÓÉŹĒ
ӣ
±ū×éĶ¬Ń§½ųŠŠĮĖøĽų£¬ĖūĆĒ½«ŅŅ×éĶ¬Ń§ŹµŃéÖŠµĆµ½µÄÄŃČܹĢĢå½ųŠŠ¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ£¬²¢
×ĘÉÕ¹ĢĢåÖĮŗćÖŲ£¬²āµĆĘäÖŹĮæĪŖ4.08g”£ÉĻŹö¹ż³ĢÖŠ£¬Ļ“µÓ³ĮµķµÄ²Ł×÷ŹĒ
ӣ
Ć¾ŌŚæÕĘųÖŠČ¼ÉÕŗóÉś³ÉµÄ¹ĢĢåÖŠMg3N2µÄÖŹĮæ·ÖŹżĪŖ ”£
£Ø4£©ÓŠŅ»ÖÖÓĆļ§ŃĪÓėŗ¬Ć¾æóŹÆ»ģŗĻģŃÉÕÖĘČ”Ńõ»ÆĆ¾µÄ·½·Ø£¬½ā¾öĮĖĻÖÓŠ·½·Ø“ęŌŚµÄŌĮĻ³É ±¾øß”¢ĻīÄæĶ¶×Ź“ó”¢ÄÜŗÄøß”¢ø±²śĘ·²»ŗĆÓƵČĪŹĢā£¬ĘäŌĄķŹĒ½«ŗ¬Ć¾æóŹÆ·Ū£Øŗ¬Ńõ»ÆĆ¾£©Óė ļ§ŃĪ»ģŗĻ,¾¹żģŃÉÕ”¢Ė®ČÜ”¢¹żĀĖ£¬µĆµ½“ÖĆ¾ŃĪČÜŅŗ£¬²¢»ŲŹÕģŃÉÕ²śÉśµÄ°±”£Š“³öÓĆ¹ĢĢå£ØNH4£©2SO4Óėŗ¬Ć¾æóŹÆ·ŪģŃÉյĻÆѧ·“Ó¦·½³ĢŹ½
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ¹ć¶«Ź”ÕŲĒģŹŠøßČż¼¶ÉĻѧʌĩĶ³Ņ»æ¼ŹŌĄķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
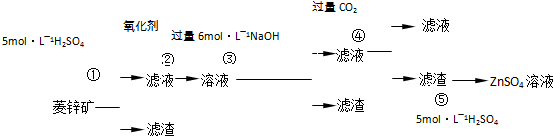
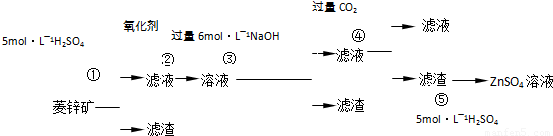
Šæ±µ°×ŹĒŅ»ÖÖ°×É«ŃÕĮĻ”£¹¤ŅµÉĻŹĒÓÉZnSO4ÓėBaSČÜŅŗ»ģŗĻ¶ų³É£ŗBaS+ZnSO4= ZnS”ż+BaSO4”ż”£Ēėøł¾ŻŅŌĻĀ¹¤ŅµÉś²śĮ÷³Ģ»Ų“šÓŠ¹ŲĪŹĢā”£
I£®ZnSO4ČÜŅŗµÄÖʱøÓėĢį“æ£ŗ
ÓŠ¹Ų׏ĮĻ£ŗa£®ĮāŠææóµÄÖ÷ŅŖ³É·ÖŹĒZnCO3£¬ŗ¬ÉŁĮæSiO2”¢FeCO3”¢Cu2(OH)2CO3µČ£»b£®Zn(OH)2ÓėAl(OH)3ĻąĖĘ£¬ÄÜČÜÓŚ¹żĮæµÄNaOHČÜŅŗÉś³ÉNa2ZnO2£»
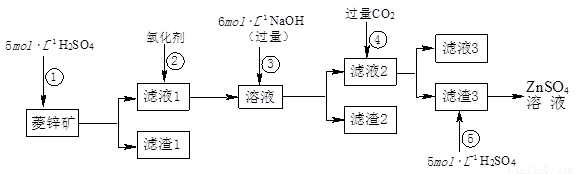
£Ø1£©ĀĖŌü1µÄ»ÆѧŹ½ĪŖ £»¢ŚÖŠŹ¹ÓƵÄŃõ»Æ¼Į×īŗĆŹĒĻĀĮŠµÄ £ØĢīŠņŗÅ£©”£
A£®Cl2 B£®H2O2 C£®KMnO4 D£®ÅØHNO3
£Ø2£©ĀĖŌü2ÖŠÖ÷ŅŖ³É·ÖµÄ»ÆѧŹ½ĪŖ £»ĪŖĮĖ“ļµ½×ŪŗĻĄūÓĆ”¢½ŚÄܼõÅŵÄÄæµÄ£¬ÉĻŹöĮ÷³Ģ²½Öč¢ÜÖŠµÄCO2æÉŅŌĄ“×ŌÓŚ²½Öč £ØŃ”Ģī¢Ł”¢¢Ś”¢¢Ū”¢¢Ż£©”£
£Ø3£©²½Öč¢Ü·¢ÉśµÄĄė×Ó·“Ó¦·½³ĢŹ½ĪŖ ”£
II£®BaSČÜŅŗµÄÖʱø
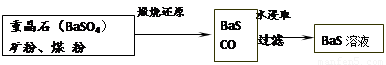
ÓŠ¹ŲŹż¾Ż£ŗBa£Øs£©£«S£Øs£©£«2O2£Øg£©£½BaSO4£Øs£© ”÷H1 = £1473.2 kJ•mol£1
C£Øs£©£« 1/2O2£Øg£©£½CO£Øg£© ”÷H2 = £110.5 kJ•mol£1
Ba£Øs£©£« S£Øs£©£½BaS£Øs£© ”÷H3 = £460 kJ•mol£1
£Ø4£©ČōģŃÉÕ»¹ŌµÄ²śĪļ½öĪŖBaSŗĶCO£¬ŌņĘä·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ
ӣ
¢ó.ÖĘČ”Šæ±µ°×
£Ø5£©Čē¹ū¢ńÖŠ²½Öč¢ŻŹ¹ÓĆĮņĖį¹żĮ棬²śÉśµÄŗó¹ūŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2009-2010ѧğ½ĖÕŹ”Õņ½ŹŠøßČż£ØÉĻ£©ĘŚÄ©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
 BaS+COӟ
BaS+CO”ü BaSČÜŅŗ
BaSČÜŅŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com