
����Ŀ��A��B��C �ֱ������ѧ��ѧ�еij������ʣ��������ĿҪ��ش��������⣺
��1��ʵ���ҳ���A�ı�����Һ�Ʊ���ֱ��Ϊ1nm-100nm�ĺ��ɫҺ���ɢϵ����÷�Ӧ�Ļ�ѧ����ʽΪ��_________________________����A ����Һ�������ɲ����գ��õ�����Ļ�ѧʽΪ��________��
��2��BΪ�ؿ��к�����ߵĽ���Ԫ�ص��Ȼ����50.0mL��4mol/L��B��Һ����ε���100mL ijŨ�ȵ�KOH��Һ��������7.8g��ɫ������������KOH��Һ��Ũ�ȿ���Ϊ_________________________��
��3����A��B�����ֽ���Ԫ�صĵ����õ������ӣ�����ͬһ��ʢ��KOH��Һ���ձ��й���ԭ��أ��������ĵ缫��ӦΪ��_____________________________________��
��4��C�dz����������壬��һ����������Cͨ��100mLijŨ�ȵ�KOH��Һ����ҺF����F��Һ����μ���2 mol��L��1�����ᣬ����CO2������������������֮���ϵ��ͼ��ʾ��
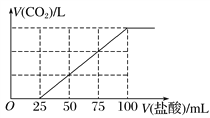
��F ����������ʯ��ˮ������Ӧ���ɵó���������Ϊ_______________g��
��5��pH��3.6ʱ��̼�������������Ӧ���Ʊ���ʽ������[Al2(SO4)x(OH)6��2x]��Һ������Һ��pHƫ�ߣ����ʽ���������ʽ�����������C�������û�ѧ����ʽ��ʾ��ԭ�� ______________��
���𰸡� FeCl3+3H2O![]() Fe(OH)3(����)+3HCl Fe2O3 3mol/L��7mol/L Al- 3e-��4OH-��AlO2һ��2H2O 15 3CaCO3��Al2(SO4)3��3H2O��2Al(OH)3��3CaSO4��3CO2��
Fe(OH)3(����)+3HCl Fe2O3 3mol/L��7mol/L Al- 3e-��4OH-��AlO2һ��2H2O 15 3CaCO3��Al2(SO4)3��3H2O��2Al(OH)3��3CaSO4��3CO2��
����������1�����ɫҺ���ɢϵ��Fe(OH)3���壬��A���Ȼ���������Һ��FeCl3������Һ�ڼ���������ˮ�����������������壬��ѧ����ʽΪFeCl3+3H2O![]() Fe(OH)3(����)+3HCl��HCl���лӷ��ԣ����ȴٽ�HCl�ӷ����Ӷ��ٽ�FeCl3ˮ�⣬����ʱ�õ�����Fe(OH)3������Fe(OH)3�õ�Fe2O3����2���ؿ��к�����ߵĽ���Ԫ����Al����BΪAlCl3��������������7.8g�������ʵ���Ϊ7.8g��78g/mol=0.1mol��������㣬��Al3++3OH����Al��OH��3����֪��KOH�����ʵ���Ϊ0.1mol��3=0.3mol�������ʵ���Ũ��Ϊ0.3mol��0.1L=3mol/L���ڼ��������ӵ����ʵ���֮�ȴ���3��1��С��4��1�����ɷ���ʽ��֪
Fe(OH)3(����)+3HCl��HCl���лӷ��ԣ����ȴٽ�HCl�ӷ����Ӷ��ٽ�FeCl3ˮ�⣬����ʱ�õ�����Fe(OH)3������Fe(OH)3�õ�Fe2O3����2���ؿ��к�����ߵĽ���Ԫ����Al����BΪAlCl3��������������7.8g�������ʵ���Ϊ7.8g��78g/mol=0.1mol��������㣬��Al3++3OH����Al��OH��3����֪��KOH�����ʵ���Ϊ0.1mol��3=0.3mol�������ʵ���Ũ��Ϊ0.3mol��0.1L=3mol/L���ڼ��������ӵ����ʵ���֮�ȴ���3��1��С��4��1�����ɷ���ʽ��֪
Al3++3OH����Al��OH��3��
0.3mol 0.9mol 0.3mol
Al��OH��3+OH����AlO2��+2H2O
��0.3-0.1��mol 0.2mol
�����ĵļ�����ʵ���Ϊ0.9mol+0.2mol=1.1mol�������ʵ���Ũ��Ϊ1.1mol��0.1L��11mol/L����3��Al��Fe��KOH��Һ����ԭ��أ�Al������������Һ�ܷ�����Ӧ��Fe������������Һ����Ӧ�����AlΪ������FeΪ�����������缫��ӦʽΪAl��3e����4OH����AlO2һ��2H2O����4��C�dz����������壬C��CO2������ͼ���֪�����������ĵ�������Һ�������25mL����˵��F��̼���ƺ�̼�����ƵĻ����Һ��̼�����ת��ΪCO2����������75mL��̼����������ʵ�����0.075L��2mol/L��0.15mol������̼ԭ���غ��֪F����������ʯ��ˮ������Ӧ���ɵó��������ʵ�����0.15mol������Ϊ0.15mol��100g/mol��15g����5����Һ��pHƫ��ʱ�������������������ͬʱ����CO2���ɣ���Ӧ�Ļ�ѧ����ʽΪ3CaCO3��Al2(SO4)3��3H2O��2Al(OH)3��3CaSO4��3CO2����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Fe+FeCl3�T3FeCl2��Ӧ��ѡ�����˵IJ��Ϻ��Լ����һ��ԭ��أ�
��1������ԭ���װ��ͼ������ͼ�б�ע���缫���ϡ��������Һ�����ƣ�
��2��д����ԭ��ص缫��Ӧʽ���������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������������ء�����������������
A. ̼��ά�������ڷɻ�����B. �ߴ�������������ʾ����
C. ������������ɫ�����Ϳ��D. ��������ˮ�ľ�����ɱ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ������������ԭ�������͵���
A. SO2����SO3��������Ҫʹ�ô���2SO2(g)+O2(g)![]() 2SO3(g)
2SO3(g)
B. 500 �����ҵ��¶ȱ����¸������ںϳɵ���ӦN2(g)+ 3H2 (g)![]() 2NH3(g) ��H<0
2NH3(g) ��H<0
C. H2��I2��HIƽ���������ѹ����ɫ����H2(g)+ I2(g)![]() 2HI(g)
2HI(g)
D. ����ʹ��ˮ���� Cl2��H2O![]() H����Cl����HClO
H����Cl����HClO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����AlCl3��Һ����εμ�NaOH��Һ���������ù����пɹ۲쵽�������ǣ� ��
A.����ɫ����ų�
B.������ɫ����ų������а�ɫ��������
C.�Ȳ�����ɫ���������������ܽ�
D.�Ȳ�����ɫ�������������ܽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�ݻ�������ܱ������г���һ����A��B���������·�Ӧ��xA(g)��2B(s) ![]() yC(g)����H<0��һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺
yC(g)����H<0��һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺

��1����A��Ũ�ȱ仯��ʾ�÷�Ӧ��0��10 min�ڵ�ƽ����Ӧ����v(A)��______________________��
��2������ͼʾ��ȷ��x��y��________��
��3��0��10 min������ѹǿ________(���������䡱��С��)��
��4���Ʋ��10 min�������߱仯�ķ�Ӧ����������______________________����16min�������߱仯�ķ�Ӧ����������________________________��
�ټ�ѹ��������A��Ũ�ȡ� ������C������ �����¢ݽ��¡� �Ӵ���
��5����ƽ����ƽ�ⳣ��ΪK1��ƽ���ƽ�ⳣ��ΪK2����K1________K2(�����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ��Ҫ����0.2mol��L��1������������Һ450mL����������ƽ��ȡ����ʱ����ƽ����(���뼰����)���� ��
A. ����4.0gB. ����3.60gC. ����4.0gD. ����0.36g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�Ͽ���CO��CO2������ȼ�ϼ״�����֪�״��Ʊ����йػ�ѧ��Ӧ�Լ��ڲ�ͬ�¶��µĻ�ѧ��Ӧƽ�ⳣ�����±���ʾ��
��ѧ��Ӧ | ƽ�ⳣ�� | �¶�/�� | |
500 | 800 | ||
��2H2(g)��CO(g) | K1 | 2.5 | 0.15 |
��H2(g)��CO2(g) | K2 | 1.0 | 2.50 |
��3H2(g)��CO2(g) | K3 | ||
��1���ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵ����K3��________����K1��K2��ʾ����500 ��ʱ��÷�Ӧ����ijʱ�̣�H2(g)��CO2(g)��CH3OH(g)��H2O(g)��Ũ��(mol��L��1)�ֱ�Ϊ0.8��0.1��0.3��0.15�����ʱv��________v��(�>������������<��)��
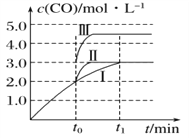
��2����3 L�ݻ��ɱ���ܱ������з�����Ӧ�ڣ���֪c(CO)����Ӧʱ��t�仯���ߢ���ͼ��ʾ������t0ʱ�̷ֱ�ı�һ�����������ߢ��Ϊ���ߢ�����ߢ����ߢ��Ϊ���ߢ�ʱ���ı��������_______________�������ߢ��Ϊ���ߢ�ʱ���ı��������______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com