
·ÖĪö £Ø1£©A£®¼×ĶéČ„µōŅ»øöĒāŌ×Ó²»ÄܵƵ½CH3+”¢CH3-£»
B£®Ō×Ó×ÜŹżĻąµČ”¢¼Ūµē×Ó×ÜŹż£Ø»ņµē×Ó×ÜŹż£©ĻąµČµÄĪ¢Į£»„ĪŖµČµē×ÓĢ壻-CH3£Ø¼×»ł£©”¢CH3-ÖŠCŌ×Ó¾łŠĪ³É3øö¦Ņ¼ü£¬¼×»łÖŠĢ¼Ō×ÓÓŠ1øöµ„µē×Ó£¬CH3-ÖŠCŌ×ÓÓɶŌ¹Ā¶Ōµē×Ó£¬ŌӻƹģµĄŹżÄæ¾łĪŖ4£»
C£®CH3-ÓėNH3”¢H3O+¾ł¾ßÓŠ4øöŌ×Ó”¢10øöµē×Ó£¬»„ĪŖµČµē×ÓĢ壬æÕ¼ä½į¹¹ĻąĖĘ£»
D£®CH3+ÖŠµÄĢ¼Ō×ÓŠĪ³É3øö¦Ņ¼ü£¬Ć»ÓŠ¹Ā¶Ōµē×Ó£¬ŌӻƹģµĄŹżÄæĪŖ3£¬ĪŖĘ½ĆęČż½ĒŠĪ½į¹¹£»
E£®Į½øö-CH3»ņŅ»øöCH3+ŗĶCH3-½įŗĻ¶¼ÄܵƵ½CH3CH3£»
£Ø2£©¢ŁZnŌ×ÓŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ1s22s22p63s23p63d104s2£»
¢ŚZnŌ×ÓŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ1s22s22p63s23p63d104s2£¬Ź§Č„4sÄܼ¶2øöµē×ÓŠĪ³ÉZn2+£»
ōČ»łÖŠCŌ×ÓŠĪ³É3øö¦Ņ¼ü£¬ĘäĖüĢ¼Ō×ÓŠĪ³É4øö¦Ņ¼ü£¬¾łĆ»ÓŠ¹Ā¶Ōµē×Ó£¬ŌӻƹģµĄŹżÄæĪŖ·Ö±šĪŖ3”¢4£»
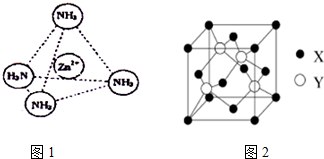
¢ŪÅäĪ»ĢåNH3·Ö×ÓĪŖČż½Ē׶ŠĪ½į¹¹£¬·Ö×ÓÖŠÕżøŗµēŗÉÖŲŠÄ²»ÖŲŗĻ£¬ŹōÓŚ¼«ŠŌ·Ö×Ó£»Zn2+Ąė×ÓÓŠæÕ¹ģµĄ£¬NH3·Ö×ÓÓŠ¹Ā¶Ōµē×Ó£¬¶žÕßĶعżÅäĪ»¼üŠĪ³ÉÅäĄė×Ó[Zn£ØNH3£©4]2+£»
¢Üøł¾Ż¾łĢƷؼĘĖć¾§°ūÖŠZnŌ×ÓŹżÄ攢XŌ×ÓŹżÄ棻
¢Ż¾§°ūÖŠX“¦ÓŚ¶„µćÓėĆęŠÄ£¬XŌ×ÓĪŖĆęŠÄĮ¢·½Ćܶѻż£»
ZnŌ×ÓÓėÖÜĪ§µÄ4øöXŌ×Ó¹¹³ÉÕżĖÄĆęĢå½į¹¹£¬ÖŠŠÄZnŌ×ÓÓė¶„µćXŌ×ÓĮ¬Ļß“¦ÓŚ¾§°ūĢå¶Ō½ĒĻßÉĻ£¬ĒŅĪŖĢå¶Ō½ĒĻß³¤¶ČµÄ$\frac{1}{4}$£¬Ōņ¾§°ūĢå¶Ō½ĒĻß³¤¶ČĪŖ4£Ør1+r2£©cm£¬¹Ź¾§°ūĄā³¤ĪŖ$\frac{4£Ø{r}_{1}+{r}_{2}£©}{\sqrt{3}}$cm£¬½įŗĻ¾§°ūÖŠŗ¬ÓŠŌ×ÓŹżÄæ¼ĘĖć¾§°ūÖŹĮ棬ŌŁøł¾Ż¦Ń=$\frac{m}{V}$¼ĘĖć¾§°ūĆÜ¶Č£®
½ā“š ½ā£ŗA£®¼×Ķé·Ö×Ó±ä³ÉCH3+”¢-CH3”¢CH3-Ź±£¬Ź§Č„µÄ·Ö±šŹĒĒāøŗĄė×Ó”¢ĒāŌ×ÓŗĶĒāĄė×Ó£¬¹ŹA“ķĪó£»
B£®CH3+”¢-CH3”¢CH3-·Ö±š¾ßÓŠ6øö”¢7øöŗĶ8øö¼Ūµē×Ó£¬²»ŹĒµČµē×ÓĢ壬-CH3£Ø¼×»ł£©”¢CH3-ÖŠCŌ×Ó¾łŠĪ³É3øö¦Ņ¼ü£¬¼×»łÖŠĢ¼Ō×ÓÓŠ1øöµ„µē×Ó£¬CH3-ÖŠCŌ×ÓÓɶŌ¹Ā¶Ōµē×Ó£¬ŌӻƹģµĄŹżÄæ¾łĪŖ4£¬¶žÕßĢ¼Ō×Ó²ÉČ”sp3Ōӻƣ¬¹ŹB“ķĪó£»
C£®CH3-ÓėNH3”¢H3O+¾ł¾ßÓŠ8øö¼Ūµē×Ó”¢4øöŌ×Ó£¬»„ĪŖµČµē×ÓĢ壬¼øŗĪ¹¹ŠĶ¾łĪŖČż½Ē׶ŠĪ£¬¹ŹCÕżČ·£»
D£®CH3+ÖŠµÄĢ¼Ō×ÓŠĪ³É3øö¦Ņ¼ü£¬Ć»ÓŠ¹Ā¶Ōµē×Ó£¬ŌӻƹģµĄŹżÄæĪŖ3£¬Ģ¼Ō×Ó²ÉČ”sp2Ōӻƣ¬ĪŖĘ½ĆęČż½ĒŠĪ½į¹¹£¬ĖłÓŠŌ×Ó“¦ÓŚĶ¬Ņ»Ę½Ćę£¬¹ŹDÕżČ·£»
E£®Į½øö-CH3»ņŅ»øöCH3+ŗĶCH3-½įŗĻ¶¼ÄܵƵ½CH3CH3£¬¹ŹEÕżČ·£»
¹ŹŃ”£ŗCDE£»
£Ø2£©¢ŁZnŌ×ÓŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ1s22s22p63s23p63d104s2£¬“¦ÓŚÖÜĘŚ±ķÖŠµŚĖÄÖÜĘŚ¢ņB×壬
¹Ź“š°øĪŖ£ŗµŚĖÄÖÜĘŚ¢ņB×壻
¢ŚZnŌ×ÓŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ1s22s22p63s23p63d104s2£¬Ź§Č„4sÄܼ¶2øöµē×ÓŠĪ³ÉZn2+£¬Zn2+»łĢ¬µē×ÓÅŲ¼Ź½ĪŖ£ŗ1s22s22p63s23p63d10£¬
ōČ»łÖŠCŌ×ÓŠĪ³É3øö¦Ņ¼ü£¬ĘäĖüĢ¼Ō×ÓŠĪ³É4øö¦Ņ¼ü£¬¾łĆ»ÓŠ¹Ā¶Ōµē×Ó£¬ŌӻƹģµĄŹżÄæĪŖ·Ö±šĪŖ3”¢4£¬·Ö×ÓÖŠĢ¼Ō×ÓŌӻƷ½Ź½ĪŖ£ŗsp2”¢sp3Ōӻƣ¬
¹Ź“š°øĪŖ£ŗ1s22s22p63s23p63d10£»sp2”¢sp3£»
¢ŪÅäĪ»ĢåNH3·Ö×ÓĪŖČż½Ē׶ŠĪ½į¹¹£¬·Ö×ÓÖŠÕżøŗµēŗÉÖŲŠÄ²»ÖŲŗĻ£¬ŹōÓŚ¼«ŠŌ·Ö×Ó£»Zn2+Ąė×ÓÓŠæÕ¹ģµĄ£¬NH3·Ö×ÓÓŠ¹Ā¶Ōµē×Ó£¬¶žÕßĶعżÅäĪ»¼üŠĪ³ÉÅäĄė×Ó[Zn£ØNH3£©4]2+£¬ČēĶ¼ĖłŹ¾£ŗ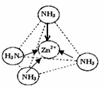 £¬
£¬
¹Ź“š°øĪŖ£ŗ¼«ŠŌ·Ö×Ó£» £»
£»
¢Ü¾§°ūÖŠZnŌ×ÓŹżÄæĪŖ4”¢XŌ×ÓŹżÄæĪŖ8”Į$\frac{1}{8}$+6”Į$\frac{1}{2}$=4£¬ZnÓėXŌ×ÓŹżÄæÖ®±ČĪŖ1£ŗ1£¬
¹Ź“š°øĪŖ£ŗ1£ŗ1£¬
¢Ż¾§°ūÖŠX“¦ÓŚ¶„µćÓėĆęŠÄ£¬XŌ×ÓĪŖĆęŠÄĮ¢·½Ćܶѻż£»
ZnŌ×ÓÓėÖÜĪ§µÄ4øöXŌ×Ó¹¹³ÉÕżĖÄĆęĢå½į¹¹£¬ÖŠŠÄZnŌ×ÓÓė¶„µćXŌ×ÓĮ¬Ļß“¦ÓŚ¾§°ūĢå¶Ō½ĒĻßÉĻ£¬ĒŅĪŖĢå¶Ō½ĒĻß³¤¶ČµÄ$\frac{1}{4}$£¬Ōņ¾§°ūĢå¶Ō½ĒĻß³¤¶ČĪŖ4£Ør1+r2£©cm£¬¹Ź¾§°ūĄā³¤ĪŖ$\frac{4£Ø{r}_{1}+{r}_{2}£©}{\sqrt{3}}$cm£¬¾§°ūÖŹĮæĪŖ
$\frac{4”Į£Ø{M}_{1}+{M}_{2}£©}{{N}_{A}}$g£¬Ōņ¾§°ūĆܶČ=$\frac{4”Į£Ø{M}_{1}+{M}_{2}£©}{{N}_{A}}$g”Ā£Ø$\frac{4£Ø{r}_{1}+{r}_{2}£©}{\sqrt{3}}$cm£©3=$\frac{3\sqrt{3}£Ø{M}_{1}+{M}_{2}£©}{16{N}_{A}£Ø{r}_{1}+{r}_{2}£©^{3}}$g/cm3£¬
¹Ź“š°øĪŖ£ŗĆęŠÄĮ¢·½Ćܶѻż£»$\frac{3\sqrt{3}£Ø{M}_{1}+{M}_{2}£©}{16{N}_{A}£Ø{r}_{1}+{r}_{2}£©^{3}}$£®
µćĘĄ ±¾ĢāŹĒ¶ŌĪļÖŹ½į¹¹ÓėŠŌÖŹµÄ漲飬Éę¼°ŗĖĶāµē×ÓÅŲ¼Ź½”¢æռ乹ŠĶÓėŌӻƷ½Ź½µÄÅŠ¶Ļ”¢µČµē×ÓĢ唢ÅäŗĻĪļ”¢¾§°ū¼ĘĖćµČ£¬¾§°ūĆÜ¶Č¼ĘĖćĪŖŅדķµć”¢ÄŃ¶Č£¬¹Ų¼üŹĒ¼ĘĖć¾§°ūĄā³¤£¬ŠčŅŖѧɜ¾ß±øŅ»¶ØµÄæÕ¼äĻėĻóÓėŹż¾Ż¼ĘĖćÄÜĮ¦£®


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ÓŠ¹ŲĪļÖŹµÄ×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£Ø²æ·ÖĪļÖŹÓėĢõ¼žŅŃĀŌČ„£©£®ŅŃÖŖAĪŖµ»ĘÉ«¹ĢĢ壬BĪŖ³£¼ūµÄĪŽÉ«ŅŗĢ壬CĪŖĮ½ÖÖŌŖĖŲ×é³ÉµÄ»ÆŗĻĪļ£¬Ä¦¶ūÖŹĮæĪŖ150g/mol£¬EĪŖµ„ÖŹĘųĢ壬FĪŖĘųĢ¬Ēā»ÆĪļ£¬ÓŠ³ō¼¦µ°ĘųĪ¶£¬GĪŖĮ½ŠŌĒāŃõ»ÆĪļ£¬KĪŖ³£¼ūµÄĖį£®
ÓŠ¹ŲĪļÖŹµÄ×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£Ø²æ·ÖĪļÖŹÓėĢõ¼žŅŃĀŌČ„£©£®ŅŃÖŖAĪŖµ»ĘÉ«¹ĢĢ壬BĪŖ³£¼ūµÄĪŽÉ«ŅŗĢ壬CĪŖĮ½ÖÖŌŖĖŲ×é³ÉµÄ»ÆŗĻĪļ£¬Ä¦¶ūÖŹĮæĪŖ150g/mol£¬EĪŖµ„ÖŹĘųĢ壬FĪŖĘųĢ¬Ēā»ÆĪļ£¬ÓŠ³ō¼¦µ°ĘųĪ¶£¬GĪŖĮ½ŠŌĒāŃõ»ÆĪļ£¬KĪŖ³£¼ūµÄĖį£®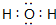 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
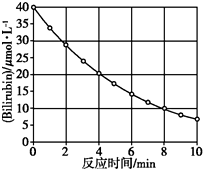 »ÆŗĻĪļBilirubinŌŚŅ»¶Ø²Ø³¤µÄ¹āÕÕÉäĻĀ·¢Éś·Ö½ā·“Ó¦£¬·“Ó¦ĪļÅضČĖę·“Ó¦Ź±¼ä±ä»ÆČēĶ¼ĖłŹ¾£¬¼ĘĖć·“Ó¦4”«8 min¼äµÄĘ½¾ł·“Ó¦ĖŁĀŹŗĶĶĘ²ā·“Ó¦16 minŹ±·“Ó¦ĪļµÄÅØ¶Č£¬½į¹ūÓ¦ŹĒ£Ø””””£©
»ÆŗĻĪļBilirubinŌŚŅ»¶Ø²Ø³¤µÄ¹āÕÕÉäĻĀ·¢Éś·Ö½ā·“Ó¦£¬·“Ó¦ĪļÅضČĖę·“Ó¦Ź±¼ä±ä»ÆČēĶ¼ĖłŹ¾£¬¼ĘĖć·“Ó¦4”«8 min¼äµÄĘ½¾ł·“Ó¦ĖŁĀŹŗĶĶĘ²ā·“Ó¦16 minŹ±·“Ó¦ĪļµÄÅØ¶Č£¬½į¹ūÓ¦ŹĒ£Ø””””£©| A£® | 2.5 ¦Ģmol/£Ø L•min£©ŗĶ2.0 ¦Ģmol/L | B£® | 2.5 ¦Ģmol/£Ø L•min£©ŗĶ2.5 ¦Ģmol/L | ||
| C£® | 3.0 ¦Ģmol/£Ø L•min£©ŗĶ3.0 ¦Ģmol/L | D£® | 5.0 ¦Ģmol/£Ø L•min£©ŗĶ3.0 ¦Ģmol/L |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £©£ŗ
£©£ŗ
 ӢF
”¢F £®
£® ”¢¢į
”¢¢į £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
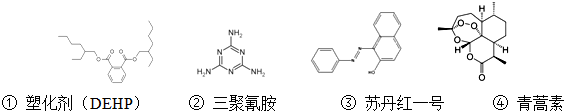
| A£® | ĖܻƼĮ£ØDEHP£©·Ö×Ó±½»·ÉĻµÄ¶žĀČ“śĪļÓŠĮłÖÖ²»Ķ¬µÄ½į¹¹ | |
| B£® | Čż¾ŪĒč°·µÄ·Ö×ÓŹ½ĪŖC3N6H6 | |
| C£® | ĖÕµ¤ŗģŅ»ŗÅ·Ö×ÓÖŠĖłÓŠŌ×ÓæÉÄÜŌŚĶ¬Ņ»Ę½ĆęÉĻ | |
| D£® | ĒąŻļĖŲÄܹ»ÖĪĮĘű¼²æÉÄÜÓė½į¹¹ÖŠ“ęŌŚ¹żŃõ¼ü»łĶÅÓŠ¹Ų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ŹµŃé | ĻÖĻó | ½įĀŪ |
| A | ÓĆ½ąQ²¬ĖæÕŗȔɣĮæ“ż²āŅŗŌŚ¾Ę¾«µĘ»šŃęÉĻ×ĘÉÕ | »šŃę³ŹĻÖ»ĘÉ« | “ż²āŅŗÖŠÖ»ŗ¬Na+£¬²»ŗ¬K+ |
| B | ÓĆĢś×÷µē¼«µē½ā±„ŗĶŹ³ŃĪĖ® | Į½¼«ÉĻ¾ł²śÉśĘųĢå | Ņõ”¢Ńō¼«·Ö±šÉś³ÉH2ŗĶCl2 |
| C | Ļņ±„ŗĶĀČĖ®ÖŠ¼ÓČĖÉŁĮæŹÆ»ŅŹÆ | ²śÉśĪŽÉ«ĘųĢå | ĖįŠŌ£ŗHCl0£¾H2CO3 |
| D | ĻņCa£ØClO£©2ČÜŅŗÖŠ ĶØČė×ćSO2 | ²śÉś°×É«³Įµķ | Ca£ØC1O£©2¾ßÓŠŃõ»ÆŠŌ£¬³ĮµķĪŖCaSO4 |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
 ÓĆĖįŠŌĒāŃõČ¼ĮĻµē³Ųµē½āæąĀ±Ė®£Øŗ¬Cl-”¢Br-”¢Na+”¢Mg2+£©µÄ×°ÖĆČēĶ¼ĖłŹ¾£Øa”¢bĪŖŹÆÄ«µē¼«£©£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
ÓĆĖįŠŌĒāŃõČ¼ĮĻµē³Ųµē½āæąĀ±Ė®£Øŗ¬Cl-”¢Br-”¢Na+”¢Mg2+£©µÄ×°ÖĆČēĶ¼ĖłŹ¾£Øa”¢bĪŖŹÆÄ«µē¼«£©£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©| A£® | µē³Ų¹¤×÷Ź±£¬B¼«·“Ó¦Ź½ĪŖO2+2H2O+4e-=4OH- | |
| B£® | µē½āŹ±£¬µē×ÓĮ÷¶ÆĀ·¾¶ŹĒ£ŗøŗ¼«”śĶāµēĀ·”śŅõ¼«”śČÜŅŗ”śŃō¼«”śÕż¼« | |
| C£® | ŹŌ¹ÜÖŠNaOHČÜŅŗÓĆĄ“ĪüŹÕµć½āŹ±²śÉśµÄCl2 | |
| D£® | µ±µē³ŲÖŠĻūŗÄ2.24L£Ø±ź×¼×“æö£©H2Ź±£¬b¼«ÖÜĪ§Ņ²»į²śÉś0.01molĘųĢå |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ca2+ | B£® | OH- | C£® | Ba2+ | D£® | NH${\;}_{4}^{+}$ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com