
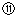 ��¼Һ��̶ȵĶ�����
��¼Һ��̶ȵĶ�����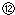 ���ݵζ��ܵ����ζ����ó�NaOH��Һ�����Ϊ22mL
���ݵζ��ܵ����ζ����ó�NaOH��Һ�����Ϊ22mL| ����NaOH��Һ�����V/mL | 0.00 | 18.00 | 19.80 | 19.98 | 20.00 | 20.02 | 20.20 | 22.00 | 40.00 |
| ʣ��������Һ�����V/mL | 20.00 | 2.00 | 0.20 | 0.02 | 0.00 | / | / | / | / |
| ����NaOH��Һ�����V/mL | / | / | / | / | / | 0.02 | 0.20 | 2.00 | 20.00 |
| pH | 1.00 | 2.28 | 3.30 | 7.00 | 9.70 | 11.70 | 12.50 |

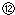 �ζ���ȷ��Ϊ0.01mL���ζ��ܵ����ζ����ó�NaOH��Һ�����Ӧ��Ϊ22.00mL����
�ζ���ȷ��Ϊ0.01mL���ζ��ܵ����ζ����ó�NaOH��Һ�����Ӧ��Ϊ22.00mL����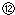 ����
����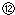 ��
��| 0.1000mol/L��0.02��10-3L |
| 0.01998L+0.020L |
| 0.1000mol/L��0.2��10-3L |
| 0.020L+0.0202L |
 ��
�� ��
��

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ף��ٳ�ȡһ��������HA����0.1 mol��L-1����Һ100 mL��
����pH��ֽ�������ҺpH������֤��HA��������ʡ�
�ң�������֪���ʵ���Ũ�ȵ�HA��Һ�����ᣬ�ֱ�����pH=1����������Һ��100 mL��
�ڷֱ�ȡ��������Һ��10 mL����ˮϡ��Ϊ100 mL��
�۸�ȡ��ͬ���������ϡ��Һװ����֧�Թܣ�ͬʱ���봿����ͬ��п�����۲�������֤��HA��������ʡ�
(1)�����������ĵڢٲ��У���Ҫ�õ��Ķ���������____________��
(2)�����У�˵��HA��������ʵ������Dz����Һ��pH_________(���������������)1���ҷ����У�˵��HA��������ʵ�������_________��
A.װHCl��Һ���Թ��зų�H2�����ʿ�
B.װHA��Һ���Թ��зų�H2�����ʿ�
C.��֧�Թ��в�����������һ����
(3)�������ۣ��ҷ���������ʵ��֮���Ͳ���֮����_____________________________��
(4)���������һ���������Ƚ������еķ���(ҩƷ����ȡ)����������Ҫ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ף��ٳ�ȡһ��������HA����0.1 mol��L-1����Һ100 mL��
����pH��ֽ�������Һ��pH������֤��HA��������ʡ�
�ң�������֪���ʵ���Ũ�ȵ�HA��Һ�����ᣬ�ֱ�����pH��1����������Һ��100 mL��
�ڷֱ�ȡ��������Һ��10 mL����ˮϡ��Ϊ100 mL��
�۸�ȡ��ͬ���������ϡ��Һװ����֧�Թܣ�ͬʱ���봿����ͬ��п�����۲�������֤��HA��������ʡ�
(1)�����������ĵڢٲ��У���Ҫ�õĶ���������______________________��
(2)�����У�˵��HA��������ʵ������Dz����Һ��pH_________1(ѡ�����������������)���ҷ����У�˵��HA��������ʵ�������________(��ѡ�۷�)���� ��
a.װHCl��Һ���Թ��зų�H2�����ʿ�
b.װHA��Һ���Թ��зų�H2�����ʿ�
c.�����Թ��в�����������һ����
(3)�������ۣ��ҷ���������ʵ��֮���Ͳ���֮��______________________��
(4)���������һ���������Ƚ������еķ���(ҩƷ����ȡ)������Ҫ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ�������ָʾ��������д���пհף�
��1���ñ���������Һ�ζ������NaOH��Һʱ,���ְ�����ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�ı仯��ֱ�������һ���������Һ�ɻ�ɫ��Ϊ��ɫ����______ _________Ϊֹ��
��2�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼʾ��������������Һ�����Ϊ___ _____ mL��
��3�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���_____________��
A.��ʽ�ζ���δ�ñ�Һ��ϴ��ֱ��ע���������Һ
B.�ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û����
C.��ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D��ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��4��ijѧ����������ʵ��ֱ��¼�й��������±���
| �ζ����� | ��������������Һ�����/mL | 0.1000mol��L-1��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
�����������ݼ����NaOH��Һ�����ʵ���Ũ�ȡ�c��NaOH��= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�差����У����-�߶���ѧ�����п��Ի�ѧ�� ���ͣ������
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ�������ָʾ��������д���пհף�
��1���ñ���������Һ�ζ������NaOH��Һʱ,���ְ�����ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�ı仯��ֱ�������һ���������Һ�ɻ�ɫ��Ϊ��ɫ����______ _________Ϊֹ��
��2�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼʾ��������������Һ�����Ϊ___ _____ mL��
��3�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���_____________��
A.��ʽ�ζ���δ�ñ�Һ��ϴ��ֱ��ע���������Һ
B.�ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û����
C.��ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D��ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��4��ijѧ����������ʵ��ֱ��¼�й��������±���
| �ζ����� | ��������������Һ�����/mL | 0.1000mol��L-1��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�差����У����-�߶���ѧ�����п��Ի�ѧ�� ���ͣ������
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ�������ָʾ��������д���пհף�
��1���ñ���������Һ�ζ������NaOH��Һʱ,���ְ�����ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�ı仯��ֱ�������һ���������Һ�ɻ�ɫ��Ϊ��ɫ����______ _________Ϊֹ��
��2�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼʾ��������������Һ�����Ϊ___ _____ mL��
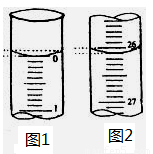
��3�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���_____________��
A.��ʽ�ζ���δ�ñ�Һ��ϴ��ֱ��ע���������Һ
B.�ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û����
C.��ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D��ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��4��ijѧ����������ʵ��ֱ��¼�й��������±���
|
����� |
��������������Һ�����/mL |
0.1000mol��L-1��������/mL |
||
|
�ζ�ǰ�̶� |
�ζ���̶� |
��Һ���/mL |
||
|
��һ�� |
25.00 |
0.00 |
26.11 |
26.11 |
|
�ڶ��� |
25.00 |
1.56 |
30.30 |
28.74 |
|
������ |
25.00 |
0.22 |
26.31 |
26.09 |
�����������ݼ����NaOH��Һ�����ʵ���Ũ�ȡ�c��NaOH��= ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com