��ҵ�ϵ�ⱥ��ʳ��ˮ����ȡ���ֻ���ԭ�ϣ����в���ԭ�Ͽ������Ʊ��ྦྷ�裮

��1��ͼ1�����ӽ���Ĥ��������������ͨ������������������������ͨ������ⱥ��ʳ��ˮ����������������������
��NaOH��Һ�ij���Ϊ
������ĸ�������Ʊ���ʳ��ˮ�Ľ���Ϊ
������ĸ������������Ӧʹ�õ�Һ����
��
��2���ྦྷ����Ҫ����SiHCl
3��ԭ�����������丱����SiCl
4���ۺ������ܵ��㷺��ע��SiCl
4���������̿�ڣ�����ά��Ҫԭ����ͬ��������Ϊ������SiCl
4��H
2��O
2��Ӧ�����������֣���ѧ����ʽΪ
��SiCl
4��ת��ΪSiCl
3��ѭ��ʹ�ã�һ�������£���20L�����ܱ������з�Ӧ3SiCl
4��g��+2H
2��g��+Si��s���T
4SiHCl
3��g����ƽ���H
2��SiHCl
3���ʵ���Ũ�ȷֱ�Ϊ0.140mol/L��0.020mol/L����H
2ȫ����Դ�����ӽ���Ĥ���ĵ����������������NaCl������Ϊ
kg��
��3����ͼ2��ʵ�����Ʊ�H
2��Cl
2ͨ���������з�Ӧ��
Zn+H
2SO
4�TZnSO
4+H
2����MnO
2+4HCl��Ũ��
MnCl
2+Cl
2��+2H
2O
�ݴˣ���������������װ���У�ͼ2��ѡ���Ʊ����ռ�H
2��װ��
������ţ����Ʊ����ռ��������C1
2��װ��
������ţ���
��4��������Ĥ���۵�ⱥ��ʳ��ˮ������ȡ�����ƣ�ͬʱ�������������Ƶ�������213.0kg������������
m
3����״�����������Կ��ܴ��ڵ�������Ӧ��




 �߽�������ϵ�д�
�߽�������ϵ�д�



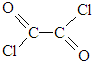 ���������л���D��Ӧ�Ļ�ѧ����ʽ
���������л���D��Ӧ�Ļ�ѧ����ʽ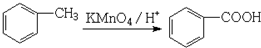

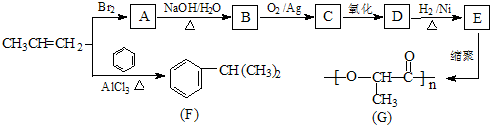
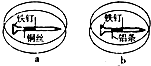 ��ͼ��ʾ�����������Ʋ�ͬ���������������������У��ټ��뺬��������̪��
��ͼ��ʾ�����������Ʋ�ͬ���������������������У��ټ��뺬��������̪��