
��ͼ�漰����������Ԫ���У���һ��Ԫ���⣬�����Ϊ������Ԫ�ء���֪��A��FΪ��ɫ���嵥�ʣ�BΪ���д̼�����ζ�����壬CΪ��ɫ�����EΪ��ɫ�������ʣ����ַ�Ӧ�IJ���δ�г�������ش��������⣺
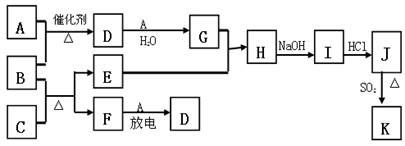
��1��B�ĵ���ʽΪ ��
��2��д��B��C��Ӧ�Ļ�ѧ����ʽ ��
��3��д��E��G��ϡ��Һ��Ӧ�����ӷ���ʽ�����õ����ű������ת�Ƶķ������Ŀ��
��
��4��J��K����ͬ�ֽ������Ȼ����KΪ��ɫ������д��SO2��ԭJ����K�����ӷ���ʽ ��
��5������β���г�����D��CO �������ڴ��������¿��Դ�ת��Ϊ���ֶԿ�������Ⱦ�����ʣ���֪�� F(g) + A(g) = 2D (g) ��H = +180.5KJ/mol
2C (s)+ O2 (g)= 2CO(g) ��H = -221.0 KJ/mol
C (s)+ O2(g) = CO2(g) ��H = -393.5 KJ/mol
������β��ת�����Ȼ�ѧ����ʽΪ�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| ||
| ||


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���ͼ�漰����������Ԫ���У���һ��Ԫ���⣬�����Ϊ������Ԫ�ء���֪��A��FΪ��ɫ���嵥�ʣ�BΪ���д̼�����ζ�����壬CΪ��ɫ�����EΪ��ɫ�������ʣ����ַ�Ӧ�IJ���δ�г�������ش��������⣺
��1��B�ĵ���ʽΪ ��
��2��д��B��C��Ӧ�Ļ�ѧ����ʽ ��
��3��д��E��G��ϡ��Һ�����ӷ���ʽ��д������ת����Ŀ�� �� ��
��4������β���г�����D��CO�������ڴ��������¿��Դ�ת��Ϊ���ֶԿ�������Ⱦ�����ʣ���֪��F(g) + A(g) = 2D (g) ����H = +180.5KJ/mol
2C (s)+ O2 (g)= 2CO(g) ����H = -221.0 KJ/mol
C (s)+ O2(g) = CO2(g)����H = -393.5 KJ/mol
������β��ת�����Ȼ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ӱ�ʡ������ѧ�߶��ڶ�ѧ�����п��Ի�ѧ���� ���ͣ������
��10�֣���ͼ�漰����������Ԫ���У���һ��Ԫ���⣬�����Ϊ������Ԫ�ء���֪��A��FΪ��ɫ���嵥�ʣ�BΪ���д̼�����ζ�����壬CΪ��ɫ�����EΪ��ɫ�������ʣ����ַ�Ӧ�IJ���δ�г�������ش��������⣺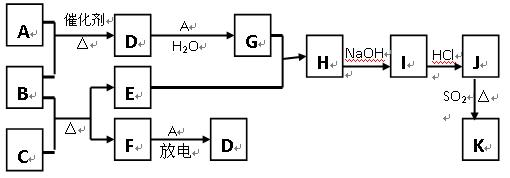
��1��B�ĵ���ʽΪ ��
��2��д��B��C��Ӧ�Ļ�ѧ����ʽ ��
��3��д��E��G��ϡ��Һ�����ӷ���ʽ��д������ת����Ŀ�� �� ��
��4������β���г�����D��CO�������ڴ��������¿��Դ�ת��Ϊ���ֶԿ�������Ⱦ�����ʣ���֪��F(g) + A(g) =" 2D" (g) ����H =" +180.5KJ/mol"
2C (s)+ O2 (g)=" 2CO(g)" ����H =" -221.0" KJ/mol
C (s)+ O2(g) = CO2(g)����H =" -393.5" KJ/mol
������β��ת�����Ȼ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������ʡ��ľ˹һ�и�����ѧ�ڵ��Ĵε��п��Ի�ѧ�Ծ� ���ͣ������
��ͼ�漰����������Ԫ���У���һ��Ԫ���⣬�����Ϊ������Ԫ�ء���֪��A��FΪ��ɫ���嵥�ʣ�BΪ���д̼�����ζ�����壬CΪ��ɫ�����EΪ��ɫ�������ʣ����ַ�Ӧ�IJ���δ�г�������ش��������⣺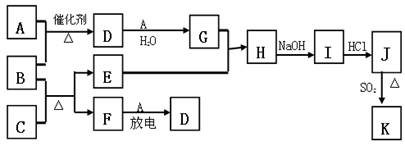
��1��B�ĵ���ʽΪ ��
��2��д��B��C��Ӧ�Ļ�ѧ����ʽ ��
��3��д��E��G��ϡ��Һ��Ӧ�����ӷ���ʽ�����õ����ű������ת�Ƶķ������Ŀ��
��
��4��J��K����ͬ�ֽ������Ȼ����KΪ��ɫ������д��SO2��ԭJ����K�����ӷ���ʽ ��
��5������β���г�����D��CO �������ڴ��������¿��Դ�ת��Ϊ���ֶԿ�������Ⱦ�����ʣ���֪�� F(g) + A(g) =" 2D" (g) ��H =" +180.5KJ/mol"
2C (s)+ O2 (g)= 2CO(g) ��H =" -221.0" KJ/mol
C (s)+ O2(g) = CO2(g) ��H =" -393.5" KJ/mol
������β��ת�����Ȼ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ӱ�ʡ�߶��ڶ�ѧ�����п��Ի�ѧ���� ���ͣ������
��10�֣���ͼ�漰����������Ԫ���У���һ��Ԫ���⣬�����Ϊ������Ԫ�ء���֪��A��FΪ��ɫ���嵥�ʣ�BΪ���д̼�����ζ�����壬CΪ��ɫ�����EΪ��ɫ�������ʣ����ַ�Ӧ�IJ���δ�г�������ش��������⣺
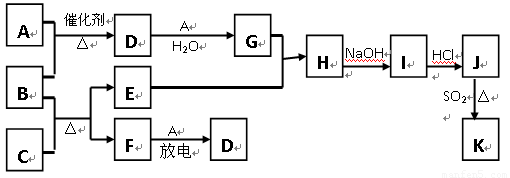
��1��B�ĵ���ʽΪ ��
��2��д��B��C��Ӧ�Ļ�ѧ����ʽ ��
��3��д��E��G��ϡ��Һ�����ӷ���ʽ��д������ת����Ŀ�� �� ��
��4������β���г�����D��CO�������ڴ��������¿��Դ�ת��Ϊ���ֶԿ�������Ⱦ�����ʣ���֪��F(g) + A(g) = 2D (g) ����H = +180.5KJ/mol
2C (s)+ O2 (g)= 2CO(g) ����H = -221.0 KJ/mol
C (s)+ O2(g) = CO2(g)����H = -393.5 KJ/mol
������β��ת�����Ȼ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com