
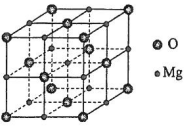
 �о����֣���CO2��ѹ�ϳɼ״���Ӧ��CO2+3H2=CH3OH+H2O���У�Co�����︺�ص�Mn�������������Ӵ������и��ԣ���ʾ�����õ�Ӧ��ǰ�����ش��������⣺
�о����֣���CO2��ѹ�ϳɼ״���Ӧ��CO2+3H2=CH3OH+H2O���У�Co�����︺�ص�Mn�������������Ӵ������и��ԣ���ʾ�����õ�Ӧ��ǰ�����ش��������⣺���� ��1��Co��27��Ԫ�أ��ɰ����������ԭ����д�����Ų�ʽ��OΪ�ǽ����ԣ�����ʧȥ���ӣ���һ�����ܽϴ�
��2��CO2��CH3OH������Cԭ�ӷֱ��γ�2��4���Ҽ���
��3��ˮ�ͼ״����Ӽ䶼���������������̼������������Ϊ���壬��������Ŀ����Է��������жϣ�
��4��Mn��NO3��2Ϊ���ӻ�����������Ӽ������ۼ������ۼ����ЦҼ��ͦм���
��5�������Ӳ��������������ܶѻ���ʽ��λ�ڶ�������ģ�������Ϊ���������ѻ������ĺ��⣬�Դ˼���뾶��
��� �⣺��1��Co��27��Ԫ�أ�λ��Ԫ�����ڱ���4���ڵ�VIII�壬���̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d74s2��[Ar]3d74s2��Ԫ��Mn��O�У�����OԪ���Ƿǽ����Զ�Mn�ǹ���Ԫ�أ����Ե�һ�����ܽϴ����O��O��̬ԭ�Ӽ۵���Ϊ2s22p4�����������δ�ɶԵ�������2����Mn��̬ԭ�Ӽ۵����Ų�Ϊ3d54s2�����������δ�ɶԵ�������5����˺���δ�ɶԵ������϶����Mn��
�ʴ�Ϊ��1s22s22p63s23p63d74s2��[Ar]3d74s2��O��Mn��
��2��CO2��CH3OH������ԭ��Cԭ�ӵļ۲���Ӷ����ֱ�Ϊ2��4������CO2��CH3OH������Cԭ�ӵ��ӻ���ʽ�ֱ�Ϊsp��sp3��
�ʴ�Ϊ��sp��sp3��
��3����CO2��ѹ�ϳɼ״���Ӧ���漰��4�������У��е�Ӹߵ��͵�˳��ΪH2O��CH3OH��CO2��H2��ԭ���dz�����ˮ�ͼ״���Һ���������̼�����������壬Һ��ķе�������壻ˮ��������������ԭ�Ӷ����Բ����γɷ��Ӽ���������״�������ֻ��һ���ǻ��ϵ���ԭ�ӿ������γɷ��Ӽ����������ˮ�ķе���ڼ״���������̼����Է������������������Զ�����̼���Ӽ��������ϴе�ϸߣ�
�ʴ�Ϊ��H2O��CH3OH��CO2��H2��������ˮ�ͼ״���Һ���������̼�����������壬Һ��ķе�������壻ˮ��������������ԭ�Ӷ����Բ����γɷ��Ӽ���������״�������ֻ��һ���ǻ��ϵ���ԭ�ӿ������γɷ��Ӽ����������ˮ�ķе���ڼ״���������̼����Է������������������Զ�����̼���Ӽ��������ϴе�ϸߣ�
��4�������������ӻ�����������������֮���γ����Ӽ����������Nԭ����3����ԭ���γ�3���Ҽ������������һ������˫�������Ի����ڦм���
�ʴ�Ϊ���м������Ӽ���
��5����ΪO2-�������������ܶѻ���ʽ����Խ�����O2-�뾶��4������4r=$\sqrt{2}$a�����r=$\frac{\sqrt{2}}{4}��0.420$nm=0.148nm��MnOҲ����NaCl�ͽṹ�����ݾ����Ľṹ����������=2 rr��O2-��+2r r��Mn2+����
��rr��Mn2+��=$\frac{0.448nm-2��0.148nm}{2}$=0.076nm��
�ʴ�Ϊ��0.148��0.076��
���� ����Ϊ2017��������⣬�漰��������Ų����������㡢�ӻ������֪ʶ�����ؿ���ѧ���ķ��������ͼ�����������Ҫѧ���������վ����ṹ���߱�һ������ѧ�����������Ѷ��еȣ�


 53������ϵ�д�
53������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����������ֱ�������Fe2O3��Fe3O4�������ȷ�Ӧʱ��ת�Ƶ��ӵ���Ŀ��� | |
| B�� | ���������ֱ�Ϊ40%��60%��������Һ�������ϣ�������Һ����������Ϊ50% | |
| C�� | �������Ż�ʱ����������ĭ�������� | |
| D�� | ϴ������ɫ��Ӧ�IJ�˿����ѡ��ϡ�����ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
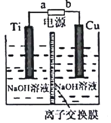
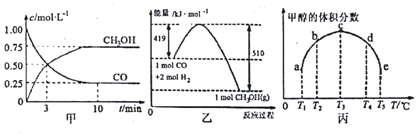
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ�� | ���� | ���� | |
| A | ��HCl��BaCl2��FeCl3��Һ | ������ɫ���� | SO2�л�ԭ�� |
| B | H2S��Һ | ������ɫ���� | SO2�������� |
| C | ����KMnO4��Һ | ��ɫ��Һ��ɫ | SO2��Ư���� |
| D | Na2SiO3��Һ | ������״���� | ���ԣ�H2SO3��H2SiO3 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ȫ��̬����������ܶȸߡ��ɱ��ͣ��乤��ԭ����ͼ��ʾ�����е缫a���ò���ʯīϩ��S8���ϣ���ط�ӦΪ��16Li+xS8=8Li2Sx��2��x��8��������˵��������ǣ�������
ȫ��̬����������ܶȸߡ��ɱ��ͣ��乤��ԭ����ͼ��ʾ�����е缫a���ò���ʯīϩ��S8���ϣ���ط�ӦΪ��16Li+xS8=8Li2Sx��2��x��8��������˵��������ǣ�������| A�� | ��ع���ʱ�������ɷ�����Ӧ��2Li2S6+2Li++2e-=3Li2S4 | |
| B�� | ��ع���ʱ�����·������0.02 mol���ӣ��������ϼ���0.14 g | |
| C�� | ʯīϩ��������Ҫ����ߵ缫a�ĵ����� | |
| D�� | ��س��ʱ��Խ��������е�Li2S2��Խ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ��Ŀ�� | ʵ����� | |
| A�� | ��ȥNaHCO3�����е�Na2CO3 | ��������������� |
| B�� | �Ʊ���ˮAlCl3 | ����Al��ϡ���ᷴӦ�����Һ |
| C�� | �ؽᾧ�ᴿ������ | ����Ʒˮ�ܡ����ˡ��������ᾧ |
| D�� | ����NaBr��KI��Һ | �ֱ��������ˮ����CCl4��ȡ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol•L-1��NH4Cl��Һ�к�NH4+����ĿС��0.01NA | |
| B�� | �÷�Ӧ�ͷ�H2�ķ�����Ϊ$\frac{V}{22.4}$NA | |
| C�� | ��Ӧ��ÿ����2molH+ת�Ƶĵ�����Ϊ6NA | |
| D�� | 120.5gNCl3���������ۼ���Ϊ4NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2O2����CO2����O2��������������߹����� | |
| B�� | ClO2���л�ԭ�ԣ�����������ˮ��ɱ������ | |
| C�� | SiO2Ӳ�ȴ�����������ά | |
| D�� | NH3������ˮ������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
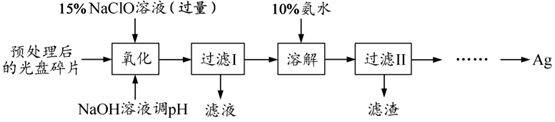
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com