��֪�������ݣ�
| ���� |
�۵㣨�棩 |
�е㣨�棩 |
�ܶȣ�g/cm3�� |
| �Ҵ� |
-117.0 |
78.0 |
0.79 |
| ���� |
16.6 |
117.9 |
1.05 |
| �������� |
-83.6 |
77.5 |
0.90 |
| Ũ���ᣨ98%�� |
- |
338.0 |
1.84 |
ѧ����ʵ������ȡ������������Ҫ�������£�
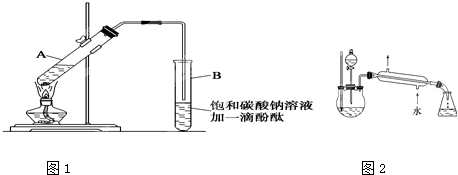
����30mL�Ĵ��Թ�A�а������2��3��2�ı�������Ũ���ᡢ�Ҵ�������Ļ����Һ��
�ڰ�ͼ1���Ӻ�װ�ã�װ�����������ã�����С����ȵؼ���װ�л����Һ�Ĵ��Թ�5-10min��
�۴��Թ�B�ռ���һ�����IJ����ֹͣ���ȣ���ȥ�Թ�B��������Ȼ���ô��ֲ㣻
�ܷ�������������㡢ϴ�ӡ����
�������ĿҪ��ش��������⣺
��1�����Ƹû����Һ����Ҫ��������Ϊ
Ӧ�ȼ����Ҵ���Ȼ���ҡ���Թܱ���������Ũ���ᣬ�����������
Ӧ�ȼ����Ҵ���Ȼ���ҡ���Թܱ���������Ũ���ᣬ�����������
��д����ȡ���������Ļ�ѧ��Ӧ����ʽ
CH
3COOH+CH
3CH
2OH
CH
3COOCH
2CH
3+H
2O
CH
3COOH+CH
3CH
2OH
CH
3COOCH
2CH
3+H
2O
��
��2������ʵ���б���̼������Һ�������ǣ�����ĸ��
BC
BC
��
A���к�������Ҵ�
B���к����Ტ���ղ����Ҵ�
C�����������ڱ���̼������Һ�е��ܽ�ȱ���ˮ�и�С�������ڷֲ�����
D�������������ɣ���������
��3�����������ҪС����ȼ��Ȳ���������Ҫ������
���������Ҵ��Ļӷ������ٸ���Ӧ�ķ���
���������Ҵ��Ļӷ������ٸ���Ӧ�ķ���
��
��4��ָ����������۲쵽������
�Թ�B�е�Һ��ֳ��������㣬�ϲ���ɫ���²�Ϊ��ɫҺ�壬���²�Һ��ĺ�ɫ��dz
�Թ�B�е�Һ��ֳ��������㣬�ϲ���ɫ���²�Ϊ��ɫҺ�壬���²�Һ��ĺ�ɫ��dz
�����������������Ϊ�˸�������������ѡ�õĸ����Ϊ������ĸ��
B
B
��
A��P
2O
5B����ˮNa
2SO
4 C����ʯ�� D��NaOH����
��5��ij��ѧ����С���������ͼ2��ʾ����ȡ����������װ�ã�ͼ�е�����̨�����С�����װ������ȥ��������ͼװ����ȣ���װ�õ���Ҫ�ŵ���
���������¶ȼƣ����ڿ��Ʒ���װ���з�ӦҺ���¶ȣ����ٸ�����ķ������������˷�Һ©���������ڼ�ʱ���䷴Ӧ���Һ����������������IJ�����������������װ�ã��������ռ���������������
���������¶ȼƣ����ڿ��Ʒ���װ���з�ӦҺ���¶ȣ����ٸ�����ķ������������˷�Һ©���������ڼ�ʱ���䷴Ӧ���Һ����������������IJ�����������������װ�ã��������ռ���������������
��




 CH3COOCH2CH3+H2O����������ˮ��
CH3COOCH2CH3+H2O����������ˮ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
 ij��ѧ��Ӧ�У��跴Ӧ���������ΪE1���������������ΪE2��
ij��ѧ��Ӧ�У��跴Ӧ���������ΪE1���������������ΪE2��
