
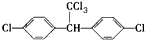
 ����DDT���ѽ���Ļ�������Բ���ʱ�䳤������������������ֹ������ʹ�á����й���DDT��˵������ȷ����
����DDT���ѽ���Ļ�������Բ���ʱ�䳤������������������ֹ������ʹ�á����й���DDT��˵������ȷ���� Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ѧ���Ƿ���ijҩ��M��������Ѫ�ܼ�������Ϊ�������������ͷų�һ�֡���ʹ���ӡ�D����������D�������ڵ�����ԭ����Ϊ�������ٻ���1998��ŵ��������ѧ��ҽѧ����
��ѧ���Ƿ���ijҩ��M��������Ѫ�ܼ�������Ϊ�������������ͷų�һ�֡���ʹ���ӡ�D����������D�������ڵ�����ԭ����Ϊ�������ٻ���1998��ŵ��������ѧ��ҽѧ����| ŨH2SO4 |



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ŨH2SO4 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������⡣
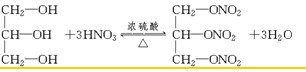
(1)��֪M����Է�������Ϊ227����C��H��O��N����Ԫ����ɣ�C��H��N��������������Ϊ15.86%��2.20%��18.50%����M�ķ���ʽ��________________��D��˫ԭ�ӷ��ӣ���Է�������Ϊ30����D�ķ���ʽΪ________________��
(2)��֬A������;���ɵõ�M��

ͼ�Тڵ���ʾ��
C2H5OH��HO��NO![]() C2H5O��NO2��H2O
C2H5O��NO2��H2O
���� ��������
��Ӧ�ٵĻ�ѧ����ʽ��__________________________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��__________________________________________________��
(3)C��B��������һ�������·�Ӧ���ɵĻ������Է�������Ϊ134��д��C���п��ܵĽṹ��ʽ��________________________________________________________________��
(4)����0.1 mol B�������Ľ����Ʒ�Ӧ����������_______________g�����ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ�ҷ���ijҩ��M��������Ѫ�ܼ�������Ϊ�������������ͷų�һ�֡���ʹ���ӡ�D����������D�������ڵ�����ԭ����Ϊ�������ٻ���1998��ŵ��������ѧ��ҽѧ����������������⡣
(1)��֪M����Է�������Ϊ227����C��H��O��N����Ԫ����ɣ�C��H��N��������������Ϊ15.86%��2.20%��18.50%����M�ķ���ʽ��________________��D��˫ԭ�ӷ��ӣ���Է�������Ϊ30����D�ķ���ʽΪ________________��
(2)��֬A������;���ɵõ�M��

ͼ�Тڵ���ʾ��
C2H5OH��HO��NO2![]() C2H5O��NO2��H2O
C2H5O��NO2��H2O
���� ��������
��Ӧ�ٵĻ�ѧ����ʽ��__________________________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��__________________________________________________��
(3)C��B��������һ�������·�Ӧ���ɵĻ������Է�������Ϊ134��д��C���п��ܵĽṹ��ʽ��________________________________________________________________��
(4)����0.1 mol B�������Ľ����Ʒ�Ӧ����������_______________g�����ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��߿���ѧһ�ָ�ϰ������ʳ�е��л�������������棩 ���ͣ������
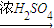 C2H5O-NO2+H2O��Ӧ�ٵĻ�ѧ����ʽ��______����Ӧ�ڵĻ�ѧ����ʽ��______��
C2H5O-NO2+H2O��Ӧ�ٵĻ�ѧ����ʽ��______����Ӧ�ڵĻ�ѧ����ʽ��______��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com