
�õ�ʯ�Ʊ�����Ȳ�����г���������H2S���塣����ͼ��������ҩƷ���һ���Ʊ���������Ȳ��װ�ã�����ͨ���ⶨ��Ȳ�������Ӷ������ʯ���ȡ�
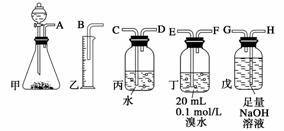
(1)����ʵ��ʱ�����������������������������ȷ����˳����________________(��ӿ���ĸ)��
(2)Ϊ��ʹʵ��������ƽ�ȣ����з�Һ©�����Һ��Xͨ����__________��
(3)���ڱ�״������ˮ����Ȳ��ȫ��Ӧ����C2H2Br4����֪��ȡ��ʯm g�������Ͳ��Һ�����V mL�����ʯ���ȿɱ�ʾΪ_________________________________��
(4)��û�г�H2S��װ�ã��ⶨ�������__________(�ƫ�ߡ�����ƫ�͡����䡱)��������________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
(1)AHGEFDCB��(2)����ʳ��ˮ��(3) %��(4)ƫ�ߡ�H2S��Br2===S����2HBr
%��(4)ƫ�ߡ�H2S��Br2===S����2HBr
������������֪����ʯ������ˮ��Ӧ������Ȳ�����л��е�H2S����NaOH��Һ���գ���Ȳ���屻��ˮ���պ����µIJ���ͨ����ˮ���������������ʯ���ȼ�����̣�
������CaC2����������C2H2
������1 mol����������1 mol
������x���������� mol��
mol�� mol
mol
���x��(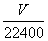 ��0.001) mol
��0.001) mol
w(CaC2)�� ��100%��
��100%�� %��
%��


 ��������������������ϵ�д�
��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ȩ�����ڵ�̼ԭ���ϵ���Ц��⡣û�Ц����ȩ��ǿ�������·������Ӽ�������ԭ��Ӧ��������ʹ��������������Ӧ�����磺2HCHO��NaOH����CH3OH��HCOONa�����л������У����ܷ����������Ӧ����(����)
A. B��
B��
C��(CH3)3C—CHO D��CH3CHO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڰ�˾ƥ�ֵĽṹ��ʽ�Т٢ڢۢܢݢֱ�����������еIJ�ͬ�ļ�������˾ƥ��������NaOH��Һ����ʱ��������Ӧʱ�ϼ���λ����(����) 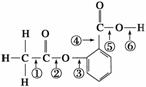
A���٢� B���ڢ�
C���ۢ� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ˮ�ֱ���ƾ�����ϩ����������Ȼ�̼�����Լ���ϣ�������ã��������������Լ������Ǻϵ���(����)
| ���� | A | B | C | D |
| ����ˮ��ϵ��Լ� | �ƾ� | ��ϩ | ���� | ���Ȼ�̼ |
| ���� |
|
|
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������������ϣ���105��ʱ1 L�������9 L O2��ϣ����ȼ�պ�ָ���ԭ״̬���������������Ϊ10 L�����и��������з��ϴ���������(����)
A��CH4��C2H4 B��CH4��C3H6
C��C2H4��C3H6 D��C2H2��C3H6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ�ݻ��̶����ܱ������н��е�ijһ���淴Ӧ��A(g)��2B(g)
 2C(g)����BŨ�ȱ�ʾ��Ӧ������ʱ��Ĺ�ϵ����֪���ʵ�λΪmol��L��1��s��1��ͼ����Ӱ�������ֵ�൱��(����)
2C(g)����BŨ�ȱ�ʾ��Ӧ������ʱ��Ĺ�ϵ����֪���ʵ�λΪmol��L��1��s��1��ͼ����Ӱ�������ֵ�൱��(����)

A��AŨ�ȵļ����� B��BŨ�ȵļ�����
C��C���ʵ����������� D��B���ʵ����ļ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ܹ�ʹ�ϳɰ���Ӧ���еij̶�����Ĵ�ʩ��(����)������������������������������������
A�������¶� B������ѹǿ C��ʹ�ô��� D��������ȥNH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪:KClO3+6HCl(Ũ) KCl+3Cl2��+3H2O,��ͼ��ʾ,�������Լ��ֱ�����������е���Ӧλ��,ʵ��ʱ��Ũ�������KClO3������,���ñ�����Ǻá��±�����
KCl+3Cl2��+3H2O,��ͼ��ʾ,�������Լ��ֱ�����������е���Ӧλ��,ʵ��ʱ��Ũ�������KClO3������,���ñ�����Ǻá��±����� ʵ������ó��Ľ�����ȫ��ȷ����(��)
ʵ������ó��Ľ�����ȫ��ȷ����(��)
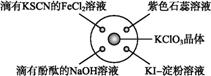
| ѡ�� | ʵ������ | ���� |
| A | ����KSCN��FeCl2��Һ��� | Cl2���л�ԭ�� |
| B | ���з�̪��NaOH��Һ��ɫ | Cl2�������� |
| C | ��ɫʯ����Һ | Cl2����Ư���� |
| D | KI | Cl2���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ʵ��װ����ɶ�Ӧ��ʵ��(����������ʡ��)���ܴﵽʵ��Ŀ�ĵ���

A.�ٸ���Cl2 B.������HCl C.��ʯ�͵����� D.������NH3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com