
£Ø10·Ö£©
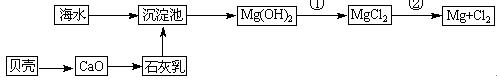
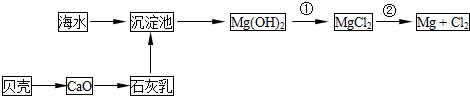
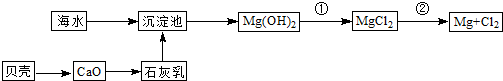
“Óŗ£Ė®ÖŠæÉŅŌ»ńµĆµĖ®”¢Ź³ŃĪ²¢æÉĢįČ”Ć¾ŗĶäåµČĪļÖŹ”£
£Ø1£©ŗ£Ė®µ»ÆµÄ·½·ØÖ÷ŅŖÓŠ £ØĢīŅ»ÖÖ£©”£
£Ø2£©¾¹ż»Æѧ±ä»Æ²ÅÄÜ“Óŗ£Ė®ÖŠ»ńµĆµÄĪļÖŹŹĒ £ØĢīŠņŗÅ£©
| A£®ĀČ”¢ä唢µā | B£®ÄĘ”¢Ć¾”¢ĀĮ | C£®ÉÕ¼ī”¢ĒāĘų | D£®Ź³ŃĪ”¢µĖ® |

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ09”«10ÄźŹÆ¼Ņ×ÆŹŠøßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»Æѧ¾ķ ĢāŠĶ£ŗŹµŃéĢā
£Ø8·Ö£©“Óŗ£Ė®ÖŠæÉŅŌ»ńµĆµĖ®”¢Ź³ŃĪ£¬²¢æÉĢįČ”Ć¾ŗĶäåµČĪļÖŹ”£
£ØŅ»£©ŗ£Ė®µ»ÆµÄ·½·ØÖ÷ŅŖÓŠ £ØĢīŅ»ÖÖ£©
£Ø¶ž£©“Óŗ£Ė®ÖŠĢįČ”äåŗĶĆ¾µÄĮ÷³ĢČēĻĀĶ¼ĖłŹ¾£ŗ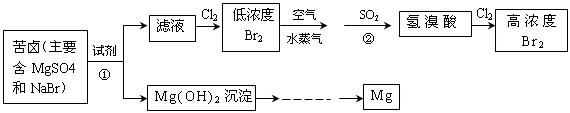
£Ø1£©ĢįČ”Br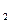 Ź±µŚŅ»“ĪĶØČėCl
Ź±µŚŅ»“ĪĶØČėCl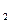 ŗó·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ £»¢ŚÖŠ±ķĻÖ
ŗó·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ £»¢ŚÖŠ±ķĻÖ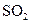 ±ķĻÖ £ØĢī”°Ńõ»Æ”±»ņ”°»¹Ō”±£©ŠŌ£»
±ķĻÖ £ØĢī”°Ńõ»Æ”±»ņ”°»¹Ō”±£©ŠŌ£»
µŚ¶ž“ĪĶØČėCl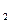 ŗó£¬ŅŖĢįČ”Br
ŗó£¬ŅŖĢįČ”Br »¹ŅŖ½ųŠŠµÄ²Ł×÷ĪŖ ”£
»¹ŅŖ½ųŠŠµÄ²Ł×÷ĪŖ ”£
£Ø2£©ĪŖĮĖŹµĻÖ¶ŌĆ¾Ąė×ÓµÄø»¼Æ£¬¢ŁÖŠ¼ÓČėµÄ×ćĮæŹŌ¼ĮŹĒ £ØĢī»ÆѧŹ½£©£»
ŹŌ“Ó½ŚŌ¼ÄÜŌ“£¬Ģįøß½šŹōĆ¾µÄ“æ¶Č·ÖĪö£¬ŅŌĻĀ×īŹŹŅĖµÄŅ±Į¶µÄ·½·ØŹĒ £ØĢī×ÖÄø£©”£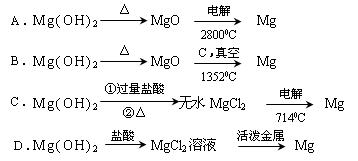
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015½ģŌĘÄĻŹ”øßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌĪÄæĘ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
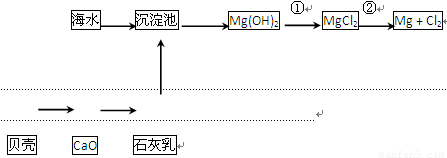
“Óŗ£Ė®ÖŠæÉŅŌ»ńµĆµĖ®”¢Ź³ŃĪ£¬²¢æÉĢįČ”Ć¾ŗĶäåµČĪļÖŹ”£
£Ø1£©ŗ£Ė®µ»ÆµÄ·½·ØÖ÷ŅŖÓŠ______£ØĢīŅ»ÖÖ£©”£
£Ø2£©“Óŗ£Ė®ÖŠĢįČ”Ć¾µÄĮ÷³ĢČēĻĀĶ¼ĖłŹ¾£ŗ
·“Ó¦¢ŁµÄĄė×Ó·½³ĢŹ½ĪŖ________________________________________________”£
·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ĪŖ________________________________________________”£
£Ø3£©“Óŗ£Ė®ÖŠĢįČ”äåµÄÖ÷ŅŖ²½ÖčŹĒĻņÅØĖõµÄŗ£Ė®ÖŠĶØČėĀČĘų£¬½«äåĄė×ÓŃõ»Æ£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com