
| |||||||||||||||
 ¶á¹Ú½ð¾íÈ«ÄÜÁ·¿¼ÏµÁдð°¸
¶á¹Ú½ð¾íÈ«ÄÜÁ·¿¼ÏµÁдð°¸
| Ä꼶 | ¸ßÖÐ¿Î³Ì | Ä꼶 | ³õÖÐ¿Î³Ì |
| ¸ßÒ» | ¸ßÒ»Ãâ·Ñ¿Î³ÌÍƼö£¡ | ³õÒ» | ³õÒ»Ãâ·Ñ¿Î³ÌÍƼö£¡ |
| ¸ß¶þ | ¸ß¶þÃâ·Ñ¿Î³ÌÍƼö£¡ | ³õ¶þ | ³õ¶þÃâ·Ñ¿Î³ÌÍƼö£¡ |
| ¸ßÈý | ¸ßÈýÃâ·Ñ¿Î³ÌÍƼö£¡ | ³õÈý | ³õÈýÃâ·Ñ¿Î³ÌÍƼö£¡ |
¿ÆÄ¿£º¸ßÖл¯Ñ§ À´Ô´£º¸£½¨Ê¡ÈªÖÝÊÐ2010Äê¸ßÖбÏÒµ°àÎåÔÂÖÊÁ¿¼ì²âÀí×Û»¯Ñ§²¿·Ö ÌâÐÍ£ºÌî¿ÕÌâ
£¨15·Ö£©
MnO2ÔÚËáÐÔÈÜÒºÖоßÓÐÇ¿Ñõ»¯ÐÔ£¬¿É±»»¹ÔΪMn2+£¬Ëü»¹¶ÔH2O2µÄ·Ö½â¾ßÓÐÁ¼ºÃµÄ´ß»¯Ð§¹û¡£Ä³ÐËȤС×éͨ¹ýʵÑéÑо¿MnO2µÄÐÔÖÊ
£¨1£©¸ÃС×éÉè¼ÆÁËÈçÏÂ4¸ö·½°¸ÒÔÑéÖ¤MnO2µÄÑõ»¯ÐÔ£¬¿ÉÐеÄÊÇ¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡£
| A£®°ÑMnO2¹ÌÌå¼ÓÈëµ½FeSO4ÈÜÒºÖУ¬ÔÙ¼ÓÈëKSCNÈÜÒº£¬¹Û²ìÈÜÒºÊÇ·ñ±äºì |
| B£®°ÑMnO2¹ÌÌå¼ÓÈëµ½FeCl3ÈÜÒºÖУ¬ÔÙ¼ÓÈëKSCNÈÜÒº£¬¹Û²ìÈÜÒºÊÇ·ñ±äºì |
| C£®°ÑMnO2¹ÌÌå¼ÓÈëµ½Na2SO3ÈÜÒºÖУ¬ÔÙ¼ÓÈëBaCl2¹Û²ìÊÇ·ñÓа×É«³ÁµíÉú³É |
| D£®°ÑMnO2¹ÌÌå¼ÓÈ뵽ϡÑÎËáÖУ¬¹Û²ìÊÇ·ñÓлÆÂÌÉ«ÆøÌåÉú³É |
| ʵÑé | Ëá»ò¼î | ÏÖÏó |
| A | 1µÎ0.2mol/LNaOHÈÜÒº | ²»±äÉ« |
| B | 1µÎË® | »ºÂý±ädz×غÖÉ« |
| C | 1µÎ0.1mol/LÁòËáÈÜÒº | ѸËÙ±ä×غÖÉ« |
 ¡¡¡¡¡¡¡¡
¡¡¡¡¡¡¡¡ ¡¡¡¡¡¡
¡¡¡¡¡¡ ¡¡¡¡
¡¡¡¡
²é¿´´ð°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖл¯Ñ§ À´Ô´£º ÌâÐÍ£ºÔĶÁÀí½â
MnO2ÔÚËáÐÔÈÜÒºÖоßÓÐÇ¿Ñõ»¯ÐÔ£¬¿É±»»¹ÔΪMn2+£¬Ëü»¹¶ÔH2O2µÄ·Ö½â¾ßÓÐÁ¼ºÃµÄ´ß»¯Ð§¹û¡£Ä³ÐËȤС×éͨ¹ýʵÑéÑо¿MnO2µÄÐÔÖÊ![]()
£¨1£©¸ÃС×éÉè¼ÆÁËÈçÏÂ4¸ö·½°¸ÒÔÑéÖ¤MnO2µÄÑõ»¯ÐÔ£¬¿ÉÐеÄÊÇ¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡£
A¡¢°ÑMnO2¹ÌÌå¼ÓÈëµ½FeSO4ÈÜÒºÖУ¬ÔÙ¼ÓÈëKSCNÈÜÒº£¬¹Û²ìÈÜÒºÊÇ·ñ±äºì
B¡¢°ÑMnO2¹ÌÌå¼ÓÈëµ½FeCl3ÈÜÒºÖУ¬ÔÙ¼ÓÈëKSCNÈÜÒº£¬¹Û²ìÈÜÒºÊÇ·ñ±äºì
C¡¢°ÑMnO2¹ÌÌå¼ÓÈëµ½Na2SO3ÈÜÒºÖУ¬ÔÙ¼ÓÈëBaCl2¹Û²ìÊÇ·ñÓа×É«³ÁµíÉú³É
D¡¢°ÑMnO2¹ÌÌå¼ÓÈ뵽ϡÑÎËáÖУ¬¹Û²ìÊÇ·ñÓлÆÂÌÉ«ÆøÌåÉú³É
£¨2£©¸ÃС×éΪÑо¿ÔÚ²»Í¬Ëá¼îÐÔµÄÈÜÒºÖÐMnO2µÄÑõ»¯ÄÜÁ¦£¬ËûÃÇ¿ØÖÆKIÈÜÒºµÄŨ¶ÈºÍMnO2¹ÌÌåµÄÖÊÁ¿Ïàͬ£¬ºã¶¨ÊµÑéζÈÔÚ298K£¬Éè¼ÆÈç϶ԱÈÊÔÑé¡£
| ʵÑé | Ëá»ò¼î | ÏÖÏó |
| A | 1µÎ0.2mol/LNaOHÈÜÒº | ²»±äÉ« |
| B | 1µÎË® | »ºÂý±ädz×غÖÉ« |
| C | 1µÎ0.1mol/LÁòËáÈÜÒº | ѸËÙ±ä×غÖÉ« |
¸ÃС×é´ÓÉÏÊö¶Ô±ÈʵÑéÖУ¬¿ÉÒԵóöµÄ½áÂÛÊÇ¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡£
д³öÔÚËáÐÔÌõ¼þÏ£¬MnO2Ñõ»¯I£µÄÀë×Ó·½³Ìʽ¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡ ¡£
£¨3£©Óû̽¾¿MnO2µÄ´ß»¯Ð§¹û£¬ÐèÒªÓÃ30%µÄH2O2ÈÜÒº£¨ÃܶȽüËÆΪ1g/cm3£©ÅäÖÆŨ¶È3%µÄH2O2ÈÜÒº£¨ÃܶȽüËÆΪ1g/cm3£©100mL¡£ÆäÅäÖÆ·½·¨ÊÇ£ºÓÃÁ¿Í²Á¿È¡¡¡¡¡¡¡mL30%H2O2ÈÜÒº·ÅÈë¡¡¡¡¡¡¡¡£¨ÌîÒÇÆ÷Ãû³Æ£©ÖУ¬ÔÙ¼ÓÈëÒ»¶¨Á¿µÄË®£¬½Á°è¾ùÔÈ¡£
£¨4£©ÔÚʵÑéʱ£¬Ä³Í¬Ñ§°Ñ1µÎKIÈÜÒºÎóµÎÈëµ½¹ýÁ¿µÄ5mL3%µÄH2O2ÈÜÒºÖУ¬·¢ÏÖ²úÉúÁË´óÁ¿ÆøÅÝ¡£¸ÃС×é²éÔĵ½KIÓëH2O2¿É·¢ÉúÈçÏ·´Ó¦£º2KI£«H2O2£½KOH£«I2£¬ÈÏΪÓпÉÄÜÊÇ·´Ó¦²úÎïI2ÆðÁË´ß»¯H2O2·Ö½âµÄ×÷Óá£ÇëÉè¼ÆÒ»¸ö¼òµ¥ÊµÑéÖ¤Ã÷¸Ã¼ÙÉèÊÇ·ñÕýÈ·¡£¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡
¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡£
£¨5£©ÊµÑéÊÒÓöþÑõ»¯Ã̺ÍŨÑÎËáÖÆÈ¡ÂÈÆø£¬ÏÂÁÐÒÇÆ÷¿É×÷Ϊ¸Ã·´Ó¦µÄ·´Ó¦ÈÝÆ÷µÄÊÇ £¨ÌîÐòºÅ£©¡£
¡¡¡¡¡¡¡¡¡¡¡¡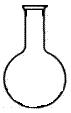 ¡¡¡¡¡¡¡¡
¡¡¡¡¡¡¡¡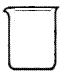 ¡¡¡¡¡¡
¡¡¡¡¡¡ ¡¡¡¡
¡¡¡¡![]()
¡¡¡¡¡¡¡¡¡¡¡¡¡¡A ¡¡ B C D
£¨6£©¶þÑõ»¯ÃÌ¿ÉÓÃÓÚÖÆ×÷¸Éµç³Ø£¬µç³Ø×Ü·´Ó¦Îª£ºZn£«2MnO2£«2NH4£«£½Zn2£«£«Mn2O3£«2NH3£«H2O£¬ÔòÆäÕý¼«µÄµç¼«·´Ó¦Ê½Îª ¡¡¡¡¡¡¡¡ ¡£
²é¿´´ð°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖл¯Ñ§ À´Ô´£º ÌâÐÍ£ºÔĶÁÀí½â
ÏÂͼΪʵÑéÊÒijŨÑÎËáÊÔ¼ÁÆ¿ÉϵıêÇ©£¬ÊÔ¸ù¾ÝÓйØÊý¾Ý»Ø´ðÏÂÁÐÎÊÌ⣺
|
£¨2£©È¡ÓÃÈÎÒâÌå»ýµÄ¸ÃÑÎËáÈÜҺʱ£¬ÏÂÁÐÎïÀíÁ¿Öв»ËæËùÈ¡Ìå»ýµÄ¶àÉÙ¶ø±ä»¯µÄÊÇ¡¡¡¡¡¡¡¡ ¡£
A£®ÈÜÒºÖÐHClµÄÎïÖʵÄÁ¿¡¡ B£®ÈÜÒºµÄŨ¶È C£®ÈÜÒºÖÐCl-µÄÊýÄ¿ D£®ÈÜÒºµÄÃܶÈ
£¨3£©Ä³Ñ§ÉúÓûÓÃÉÏÊöŨÑÎËáºÍÕôÁóË®ÅäÖÆ500 mLÎïÖʵÄÁ¿Å¨¶ÈΪ0.3 mol/LÏ¡ÑÎËá¡£
¢Ù¸ÃѧÉúÐèÒªÁ¿È¡¡¡¡¡¡¡ mLÉÏÊöŨÑÎËá½øÐÐÅäÖÆ¡£
¢ÚÅäÖƹý³ÌÖУ¬ÐèҪʹÓõÄÒÇÆ÷ÊÇ£¨Ìîд´úºÅ£©________¡£
A£®ÉÕ±¡¡¡¡ B£®Á¿Í²¡¡¡¡¡¡¡¡¡¡¡¡ C£®1000 mLÈÝÁ¿Æ¿¡¡¡¡ D£®ÍÐÅÌÌìƽ¡¡
E£®Ò©³×¡¡¡¡ F£®500 mLÈÝÁ¿Æ¿¡¡¡¡ G£®½ºÍ·µÎ¹Ü¡¡¡¡¡¡¡¡ H£®²£Á§°ô
¢ÛÅäÖÆʱ£¬ÆäÕýÈ·µÄ²Ù×÷˳ÐòÊÇ£¨×Öĸ±íʾ£¬Ã¿¸ö×ÖĸֻÄÜÓÃÒ»´Î£©¡¡¡¡¡¡¡¡¡¡¡¡ £»
A£®ÓÃ30mLˮϴµÓÉÕ±2¡ª3´Î£¬Ï´µÓÒº¾ù×¢ÈëÈÝÁ¿Æ¿£¬Õñµ´
B£®ÓÃÁ¿Í²×¼È·Á¿È¡ËùÐèŨÑÎËáµÄÌå»ý£¬ÂýÂýÑر±Ú×¢ÈëÊ¢ÓÐÉÙÁ¿Ë®£¨Ô¼30mL£©µÄÉÕ±ÖУ¬Óò£Á§°ôÂýÂý½Á¶¯£¬Ê¹Æä»ìºÏ¾ùÔÈ
C£®½«ÒÑÀäÈ´µÄÑÎËáÑز£Á§°ô×¢Èë500mLµÄÈÝÁ¿Æ¿ÖÐ
D£®½«ÈÝÁ¿Æ¿¸Ç½ô£¬µßµ¹Ò¡ÔÈ
E£®¸ÄÓýºÍ·µÎ¹Ü¼ÓË®£¬Ê¹ÈÜÒº°¼ÃæÇ¡ºÃÓë¿Ì¶ÈÏßÏàÇÐ
F£®¼ÌÐøÍùÈÝÁ¿Æ¿ÄÚСÐļÓË®£¬Ö±µ½ÒºÃæ½Ó½ü¿Ì¶ÈÏß1¡ª2cm´¦
¢ÜÔÚÅäÖƹý³ÌÖУ¬ÏÂÁÐʵÑé²Ù×÷»áʹËùÅäÖƵÄÏ¡ÑÎËáµÄÎïÖʵÄÁ¿Å¨¶ÈÆ«¸ßµÄÊÇ¡¡¡¡
A£®ÓÃÁ¿Í²Á¿È¡Å¨ÑÎËáʱ¸©Êӹ۲찼ҺÃæ
B£®ÈÜҺעÈëÈÝÁ¿Æ¿Ç°Ã»Óлָ´µ½ÊÒξͽøÐж¨ÈÝ
C£®¶¨ÈÝʱÑöÊӿ̶ÈÏß¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡ D£®ÔÚÅäÖÃÇ°ÓÃÒÑ֪Ũ¶ÈµÄÏ¡ÑÎËáÈóÏ´ÈÝÁ¿Æ¿
£¨4£©ÈôÔÚ±ê×¼×´¿öÏ£¬½«VLHClÆøÌåÈÜÓÚ1LË®ÖУ¬ËùµÃÈÜÒºÃܶÈΪd g/mL£¬Ôò´ËÈÜÒºµÄÎïÖʵÄÁ¿Å¨¶ÈΪ ¡¡¡¡ mol/L¡£

£¨5£©ÏÖ½«100mL0.5mol/LµÄÑÎËáºÍ200mL0.1mol/LCuCl2ÈÜÒº»ìºÏ£¬Ìå»ý±ä»¯ºöÂÔ²»¼Æ£¬ËùµÃÈÜÒºÖÐCl-µÄÎïÖʵÄÁ¿Å¨¶ÈÊÇ¡¡¡¡¡¡¡¡¡¡¡¡¡¡ ¡£
²é¿´´ð°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖл¯Ñ§ À´Ô´£º ÌâÐÍ£ºÔĶÁÀí½â
ϱíÁгöÁ˼¸ÖÖÎïÖʵÄÎïÀíÐÔÖÊÊý¾Ý£¬Çë²Î¿¼Ïà¹ØÎïÖʵÄÊý¾ÝºÏ³É1£ä嶡Í顣ʵÑéÊÒÖƱ¸1¡ªä嶡ÍéµÄ·´Ó¦ÈçÏ£º¢ÙNaBr + H2SO4 ?£½ HBr + NaHSO4 £»¢ÚR¡ªOH + HBr ![]() R¡ªBr +H2O¿ÉÄÜ´æÔڵĸ±·´Ó¦ÓУº¼ÓÈȹý³ÌÖз´Ó¦»ìºÏÎï»á³ÊÏÖ»ÆÉ«»òºì×ØÉ«£»´¼ÔÚŨÁòËá´æÔÚÏÂÍÑË®Éú³ÉÏ©ºÍÃѵȡ£Çë»Ø´ðÏÂÁÐÎÊÌ⣺
R¡ªBr +H2O¿ÉÄÜ´æÔڵĸ±·´Ó¦ÓУº¼ÓÈȹý³ÌÖз´Ó¦»ìºÏÎï»á³ÊÏÖ»ÆÉ«»òºì×ØÉ«£»´¼ÔÚŨÁòËá´æÔÚÏÂÍÑË®Éú³ÉÏ©ºÍÃѵȡ£Çë»Ø´ðÏÂÁÐÎÊÌ⣺
Õý¶¡´¼ | 1£ä嶡Íé | Õý¶¡ÃÑ | |
ÈÛµã/¡æ | -89.53 | -112.4 | -98 |
·Ðµã/¡æ | 117.7 | 101.6 | 142 |
ÃܶÈ/g.cm-3 | 0.8098 | 1.2758 | 0.769 |
Ë®ÈÜÐÔ | ΢ÈÜ | ²»ÈÜ | ²»ÈÜ |
ʵÑéÒ»£ºÊµÑéÊÒÖÆÈ¡ÉÙÁ¿1£ä嶡ÍéµÄ×°ÖÃÈçͼËùʾ¡£ÔÚaÖУ¬¼ÓÈë7.0mlÕý¶¡´¼¡¢×ãÁ¿µÄä廯ÄƺÍ1:1µÄÁòËá¡£
£¨1£©Ð´³ö×°ÖÃͼÖв£Á§ÒÇÆ÷µÄÃû³Æ£ºa ¡¡¡¡¡ø¡¡¡¡ £¬b¡¡¡¡ ¡ø¡¡¡¡ ¡£
£¨2£©ÅäÖÆÌå»ý±È1£º1µÄÁòËáËùÓõĶ¨Á¿ÒÇÆ÷Ϊ¡¡¡¡ ¡ø¡¡¡¡ £¨Ìî×Öĸ£©¡£
¡¡ A£®Ììƽ¡¡¡¡ ¡¡¡¡ B£®Á¿Í² ¡¡¡¡¡¡¡¡¡¡C£®ÈÝÁ¿Æ¿
£¨3£©ÖƱ¸²Ù×÷ÖУ¬¼ÓÈëµÄŨÁòËá±ØÐë½øÐÐÊʵ±µÄÏ¡ÊÍ£¬ÆäÄ¿µÄÊÇ ¡¡ ¡ø¡¡ £¨Ìî×Öĸ£©¡£
A£®¼õÉÙ¸±²úÎïÏ©ºÍÃѵÄÉú³É¡¡¡¡¡¡¡¡ ¡¡¡¡¡¡¡¡¡¡ B£®¼õÉÙBr2µÄÉú³É
C£®¼õÉÙHBrµÄ»Ó·¢¡¡¡¡¡¡¡¡¡¡¡¡¡¡ ¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡¡ D£®Ë®ÊÇ·´Ó¦µÄ´ß»¯¼Á

ʵÑé¶þ£ºÏÈÀûÓÃͼ¼××°ÖðÑÖƱ¸µÃµ½µÄ´Ö²úƷˮϴ£¬Ë®Ï´Ê±Ðè¼ÓÈë̼ËáÄƹÌÌå¼ÓÒÔÌá´¿£»ÔÙÀûÓÃͼÒÒ×°ÖýøÐÐÕôÁóÌá´¿¡£
|
|
 ¡¡
¡¡ £¨4£©ÓÃͼ¼××°ÖýøÐÐˮϴÌᴿʱ£¬ÍùÍùÒª¼ÓÈëNa2CO3¹ÌÌ壬Æä×÷ÓÃÖ÷ÒªÊdzýÈ¥¡¡¡¡ ¡ø¡¡¡¡ ¡£ÔÚͼÒÒ×°ÖÃÖмÓÈëÊʵ±µÄ¸ÉÔï¼Á£¬²¢½«Ï´µÓºóµÄ´Ö²úƷͨ¹ý·ÖҺ©¶·×ªÒƵ½Í¼ÒÒ×°ÖÃÖнøÐÐÕôÁó¡£ÏÂÁпÉ×öΪ´Ö²úÆ·ÕôÁóÌᴿʱµÄ¸ÉÔï¼ÁµÄÊÇ¡¡¡¡ ¡ø¡¡¡¡ ¡££¨Ìî×Öĸ£©
A£®NaOH¹ÌÌå¡¡¡¡¡¡ B£®¼îʯ»Ò ¡¡¡¡C£®ÎÞË®ÁòËáÄÆ¡¡¡¡¡¡¡¡ D£®ÎÞË®ÂÈ»¯¸Æ
£¨5£©ÓÃͼÒÒ×°ÖýøÐÐÕôÁóÌᴿʱ£¬µ±Î¶ȼÆÏÔʾ¡¡¡¡ ¡ø¡¡¡¡ ʱ£¬ÊÕ¼¯1£ä嶡Íé¡£
£¨6£©Èô»ñµÃÁË1£ä嶡ÍéΪ7.0¿Ë£¬ÊÔ¼ÆËãÆä²úÂÊ¡¡¡¡ ¡ø¡¡¡¡ ¡£
²é¿´´ð°¸ºÍ½âÎö>>
°Ù¶ÈÖÂÐÅ - Á·Ï°²áÁбí - ÊÔÌâÁбí
ºþ±±Ê¡»¥ÁªÍøÎ¥·¨ºÍ²»Á¼ÐÅÏ¢¾Ù±¨Æ½Ì¨ | ÍøÉÏÓк¦ÐÅÏ¢¾Ù±¨×¨Çø | µçÐÅթƾٱ¨×¨Çø | ÉæÀúÊ·ÐéÎÞÖ÷ÒåÓк¦ÐÅÏ¢¾Ù±¨×¨Çø | ÉæÆóÇÖȨ¾Ù±¨×¨Çø
Î¥·¨ºÍ²»Á¼ÐÅÏ¢¾Ù±¨µç»°£º027-86699610 ¾Ù±¨ÓÊÏ䣺58377363@163.com