
| 1 |
| 2 |
| 1 |
| 2 |


 金钥匙试卷系列答案
金钥匙试卷系列答案科目:高中化学 来源: 题型:
 请根据所提供的试剂和仪器,按要求填空:
请根据所提供的试剂和仪器,按要求填空:

查看答案和解析>>
科目:高中化学 来源: 题型:





 +O2
+O2| Cu |
| △ |
 +2H2O
+2H2O +O2
+O2| Cu |
| △ |
 +2H2O
+2H2O
 为原料合成重要的化工产品
为原料合成重要的化工产品 .合成路线流程图示例如下:
.合成路线流程图示例如下:


查看答案和解析>>
科目:高中化学 来源: 题型:
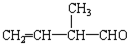 所含官能团的名称是
所含官能团的名称是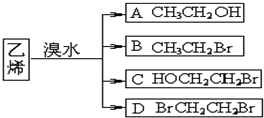
查看答案和解析>>
科目:高中化学 来源: 题型:
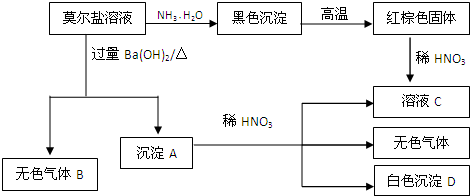
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com