

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 �ܼ�β���������ֲ��ػ�������Ҫ���ȵ������·��á����)��������������ÿ�������·������ĸB��C������������ѡ�õ�����(��������)���ױ�ʾ���£�
�ܼ�β���������ֲ��ػ�������Ҫ���ȵ������·��á����)��������������ÿ�������·������ĸB��C������������ѡ�õ�����(��������)���ױ�ʾ���£�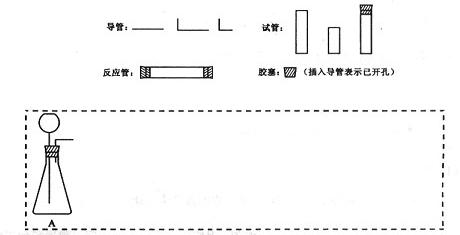
| �������� | �������������� | ���� |
| A | ʯ��ʯ��ϡ���� | ʯ��ʯ����������CO2 |
| | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

 |
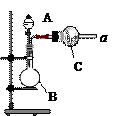
 ��Na2CO3��������A�м���ϡ���ᣬB�м���Na2CO3��NaCl�������C�м����ʯ�ҡ���װ�ô��ڽ϶�ȱ�ݣ��Ӷ�����ʵ�����ϴ�����˵�����е�����ȱ�ݣ�
��Na2CO3��������A�м���ϡ���ᣬB�м���Na2CO3��NaCl�������C�м����ʯ�ҡ���װ�ô��ڽ϶�ȱ�ݣ��Ӷ�����ʵ�����ϴ�����˵�����е�����ȱ�ݣ�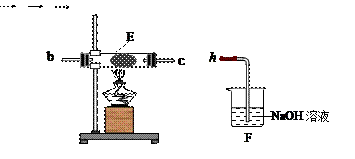 a b c h��
a b c h���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
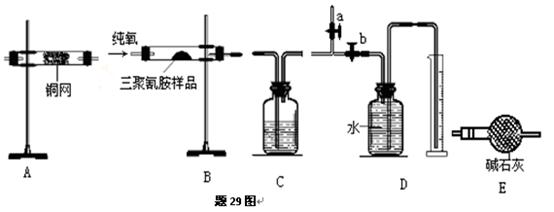
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
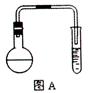
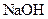 �ζ�����̪��ָʾ�������յ�ʱ����
�ζ�����̪��ָʾ�������յ�ʱ����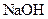 ��Һ�����Ϊ40.0mL;
��Һ�����Ϊ40.0mL;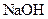 ��Һ��Ͼ��Ⱥ�װ�������ܣ���ˮԡ�ϼ��Ȼ���Լ1Сʱ��װ����ͼ��ʾ������ȴ����0.50mol/L
��Һ��Ͼ��Ⱥ�װ�������ܣ���ˮԡ�ϼ��Ȼ���Լ1Сʱ��װ����ͼ��ʾ������ȴ����0.50mol/L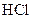 �ζ�������
�ζ������� ���յ�ʱ������������Ϊ20.0ml���ش�
���յ�ʱ������������Ϊ20.0ml���ش�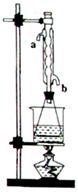
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
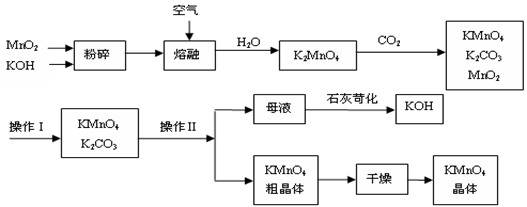
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
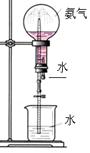
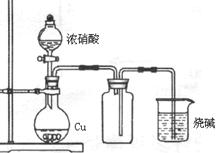
| A����������ˮ����Ȫʵ�� | B��ͭ��Ũ�����Ʊ�NO2���� 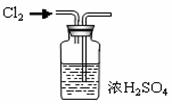 |
| C�������Ȼ���Ʊ����� | D����Ũ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ƽ����ij����ʱ�����̷����ʣ����̷����룬����������� |
| B������һ�����ʵ���Ũ����Һ������ʱ���ӿ̶��ߣ�������ҺŨ�� |
| C���ⶨ����ͭ����ᾧˮ����ʱ���ڿ�������ȴ���������ýᾧˮ���� |
| D������֪Ũ�ȵ�����ζ�δ֪Ũ�ȵ�NaOH��Һʱ����ʽ�ζ���������ˮϴ����ֱ��װ��������еζ�����ü�ҺŨ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ü���ʼ���ijKI��Һ���Ƿ����е��� |
| B������������ˮ����ˣ��ɳ�ȥ���е��������� |
| C�����ˮ�м��뱽��ƾ������ɴӵ�ˮ����ȡ�� |
| D����AgCl�����еμ�����KI��Һ����˵��Ksp(AgCl)��Ksp (AgI) |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com