
 ���������б��18O��λ�ã�
���������б��18O��λ�ã�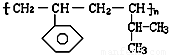

 CH3CO18OCH2CH3+H2O��
CH3CO18OCH2CH3+H2O�� CH3CO18OCH2CH3+H2O��
CH3CO18OCH2CH3+H2O��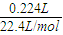 =0.01mol�����ݵ���ת���غ��֪ת�Ƶĵ��ӵ����ʵ���Ϊ0.01mol×2=0.02mol���ʵ�Դ���ṩ�ĵ���Ϊ0.02mol×6.02×1023mol-1×Q=1.204×1022Q��
=0.01mol�����ݵ���ת���غ��֪ת�Ƶĵ��ӵ����ʵ���Ϊ0.01mol×2=0.02mol���ʵ�Դ���ṩ�ĵ���Ϊ0.02mol×6.02×1023mol-1×Q=1.204×1022Q��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
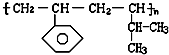
 ��1��ijʵ��С������ͼװ����ȡ������������ش��������⣺
��1��ijʵ��С������ͼװ����ȡ������������ش��������⣺
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
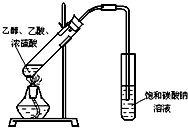
 ij��ѧʵ��С������ͼ��ʾ��װ����ȡ������������������ �� �� �����Ƿ����������ʣ�����̨�����ӵ�֧������ʡ�ԣ�����֪���������ķе�Ϊ77.1�棬�Ҵ��е�Ϊ78.4�棬����ķе�Ϊ118�森�����Ҫ����գ�
ij��ѧʵ��С������ͼ��ʾ��װ����ȡ������������������ �� �� �����Ƿ����������ʣ�����̨�����ӵ�֧������ʡ�ԣ�����֪���������ķе�Ϊ77.1�棬�Ҵ��е�Ϊ78.4�棬����ķе�Ϊ118�森�����Ҫ����գ� CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O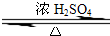 CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���1��ijʵ��С������ͼװ����ȡ������������ش��������⣺
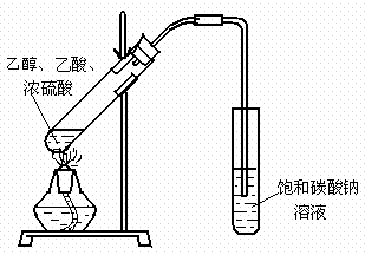
�ٸ�װ����һ��������ָ������ ����˵���ô����������ĺ���� ��
����֤����ʵ���������ʣ��������������������Ժ�ֱ�ӵ������� ��
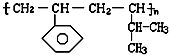
����ɸ÷�Ӧ�Ļ�ѧ����ʽ CH3CH218OH + CH3COOH![]() �� ���������б��18O��λ�ã�
�� ���������б��18O��λ�ã�
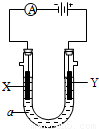
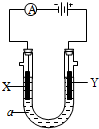
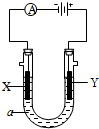
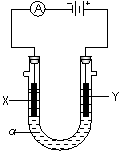 ��2�����ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾһ�����أ�װ�е��Һa��a��CuCl2��Һ��X��Y����ʯī�缫��ͨ��������ֱ����Դ������
��2�����ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾһ�����أ�װ�е��Һa��a��CuCl2��Һ��X��Y����ʯī�缫��ͨ��������ֱ����Դ������
��X���� ����������������������, X��������
��Y���ϵĵ缫��Ӧʽ ��
������Ӧ��������Y�����ռ�������224mL����״��������˹����е�Դ���ṩ�ĵ����� ������֪ÿ�����Ӵ��ĵ���ΪQ���ú�Q��ʽ�ӱ�ʾ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com