

 l/L��0.250L��40g/mol=4.02g
l/L��0.250L��40g/mol=4.02g


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ����ˮΪ�Լ�, ���Խ������Ҵ�����ϩ�����Ȼ�̼����Һ�����ֿ����� |
| B������ˮ�Ҵ����ȵ� 170��ʱ, �����Ƶ���ϩ |
| C�����Ӻ��������ڷ�ˮԡ�м��ȿ�����ȡ��ȩ��֬�� |
| D���Ҵ���������� 2 mol/L ����������, ���ȿ����Ʊ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
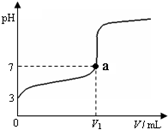
| A������ CH3COOH��Һ�У�c(H+)��1��10��3 mol��L��1 |
| B��ͼ��V1 ��20 mL |
| C��a���Ӧ����Һ�У�c (CH3COO��)��c (Na+�� |
| D��������NaOH��Һ�����Ϊ20 mLʱ����Һ�У�c (CH3COOH) + c (H+)��c (OH���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
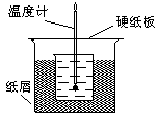
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ñ���ʳ��ˮ��������Һ�ͷ���ˮ |
| B�����Ӻͼ�ȩ��Ũ����������¿ɵõ����͵ķ�ȩ��֬ |
| C��Ϊ��С�к͵ζ�����ƿ����ϴ������ɺ����ʹ�� |
| D��������AlCl3��Һ��Al(OH)3����������ɲ����գ�������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ζ����¶�������Ƥ�ܵ�Ϊ��ʽ�ζ��� |
| B������֪���ʵ���Ũ�ȵ�����ζ�δ֪���ʵ���Ũ�ȵļ���Һ��������ָʾ��������¼��Һ��ʼ���ʱ�����Ӷ������յ�ʱ���Ӷ������������ƫ�ߡ� |
| C���ζ�ǰӦ�����ų����첿�ֵ����ݡ� |
| D���ζ�����������Ӧע�ӵζ�����Һ��ı仯�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A���ζ�ʱ��װNaOH�� Һ����ƿδ��NaOH��Һ�� Һ����ƿδ��NaOH��Һ�� ϴ ϴ |
| B����ʽ�ζ���������ˮϴ���ñ�������ϴ |
| C���ζ�ǰ����ʽ�ζ��ܼ��촦�����ݣ����ڵζ���������ʧ |
| D���ζ�ǰ���Ӷ�ȡ����ʽ�ζ��ܵĶ������ζ��������ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ӵ�ˮ����ȡ���ʵ�ʱ��������ˮ�Ҵ�����CCl4 |
| B����pH��ֽ�ⶨ��Һ��pHʱ������������ˮ��ʪ��ֽ |
| C�����ζ�ʱ�����������Һǰ�ô���Һ��ϴ��ƿ�������²ⶨ���ƫ�� |
| D������ij��Һ�Ƿ���SO42��ʱ��Ӧȡ��������Һ�����μ���BaCl2��Һ��ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����25 mL��Ͳ��ȡ12.36 mL���� |
| B����������ƽ��ȡ8.75 gʳ�� |
| C���ù㷺pH��ֽ���ij��Һ��pHΪ3.5 |
| D���ñ�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��ȥNaOH��Һ23.10 mL |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com