
| A£® | ¹āµ¼ĻĖĪ¬µÄÖ÷ŅŖ³É·ÖŹĒ¶žŃõ»Æ¹č£¬ĘäÖ÷ŅŖŌĄķŹĒ¶žŃõ»Æ¹č¾ßÓŠ½ĻŗƵĵ¼¹āŠŌ | |
| B£® | ŃĪĀ±µć¶¹øÆ”¢Ć÷·Æ¾»Ė®Óė½ŗĢåµÄŠŌÖŹÓŠ¹Ų | |
| C£® | ŠĀŠĶĒŻĮ÷øŠ²”¶¾H5N1ŠĶ²”¶¾ÖĀĖĄĀŹøߣ¬ĶعżøßĪĀĢõ¼žĻĀæÉɱĖĄH5N1ŠĶĒŻĮ÷øŠ²”¶¾ | |
| D£® | Īķö²³ÉĪŖĻÖ“śÉś»īÖŠÖ÷ŅŖµÄĪŪČ¾Ö®Ņ»£¬ĘäÖŠPM2.5Öø±źÖøµÄŹĒ·ÖÉ¢ÖŹÖ±¾¶ŌŚ2.5nmµÄĪ¢Į£ |
·ÖĪö A£®ŅĄ¾Ż¶žŃõ»Æ¹č¾ßÓŠĮ¼ŗƵĵ¼¹āŠŌ½ā“š£»
B£®ŃĪĀ±µć¶¹øÆ”¢Ć÷·Æ¾»Ė®Éę¼°µ½½ŗĢåµÄ¾Ū³ĮµČŠŌÖŹ£»
C£®øßĪĀÄܹ»Ź¹µ°°×ÖŹ±äŠŌ£»
D£®PM2.5ŹĒÖø“óĘųÖŠÖ±¾¶Š”ÓŚ»ņµČÓŚ2.5Ī¢Ć×µÄæÅĮ£Īļ£®
½ā“š ½ā£ŗA£®¶žŃõ»Æ¹č¾ßÓŠĮ¼ŗƵĵ¼¹āŠŌ£¬ŹĒÖĘ×÷¹āµ¼ĻĖĪ¬µÄÖ÷ŅŖ³É·Ö£¬¹ŹAÕżČ·£»
B£®ŃĪĀ±µć¶¹øÆ£¬ĄūÓĆĮĖ½ŗĢåµÄ¾Ū³ĮµÄŠŌÖŹ£¬Ć÷·ÆĖ®½āæÉÉś³É¾ßÓŠĪüø½ŠŌµÄĒāŃõ»ÆĀĮ½ŗĢ壬¹ŹBÕżČ·£»
C£®Ļø¾śŹōÓŚŅ»ÖÖµ°°×ÖŹ£¬øßĪĀÄܹ»Ź¹Ęä±äŠŌ£¬Ź§Č„ÉśĄķ»īŠŌ£¬¹ŹCÕżČ·£»
D£®PM2.5ŹĒÖø“óĘųÖŠÖ±¾¶Š”ÓŚ»ņµČÓŚ2.5Ī¢Ć×µÄæÅĮ£Īļ£¬¹ŹD“ķĪó£»
¹ŹŃ”£ŗD£®
µćĘĄ ±¾Ģāæ¼²éĮĖ¶žŃõ»Æ¹čµÄŠŌÖŹ”¢½ŗĢåµÄŠŌÖŹ”¢µ°°×ÖŹµÄŠŌÖŹ”¢³£¼ūÉś»ī»·¾³µÄĪŪČ¾ÓėÖĪĄķ£¬ŹģĻ¤pM2.5µÄŗ¬ŅåŹĒ½āĢā¹Ų¼ü£¬ĢāÄæÄŃ¶Č²»“ó£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | ÉżøßĪĀ¶Č£¬v£ØÕż£©”¢v£ØÄę£©¶¼Ōö“󣬵«v£ØÕż£©ŌöµÄøü“ó | |
| B£® | Ōö“óŃ¹Ē棬v£ØÕż£©”¢v£ØÄę£©¶¼Ōö“󣬵«v£ØÕż£©ŌöµÄøü“ó | |
| C£® | Ōö“óAµÄÅØ¶Č£¬v£ØÕż£©»įŌö“󣬵«v£ØÄę£©»į¼õŠ” | |
| D£® | ²ÉÓĆŹŹµ±“߻ƼĮ£ØÕż£©£¬v£ØÕż£©”¢v£ØÄę£©Ķ¬Ź±Ōö“󣬶ųĒŅŌö“óµÄ±¶ŹżĻąĶ¬ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ĪļÖŹ | CH3OH | CH3OCH3 | H2O |
| ÅضČ/£Ømol/L£© | 0.44 | 0.6 | 0.6 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
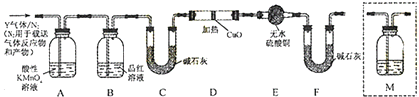
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ×īĶā²ćµē×ÓÅŲ¼ĪŖ1s2µÄŌ×ÓŗĶ×īĶā²ćµē×ÓÅŲ¼ĪŖ2s2µÄŌ×Ó | |
| B£® | 2p¹ģµĄÉĻÓŠ2øöĪ“³É¶Ōµē×ÓµÄŌ×ÓŗĶ3p¹ģµĄÉĻÓŠ2øöĪ“³É¶Ōµē×ÓµÄŌ×Ó | |
| C£® | 3p¹ģµĄÉĻÖ»ÓŠ1øöæÕ¹ģµĄµÄŌ×ÓŗĶ4p¹ģµĄÉĻÖ»ÓŠ1øöæÕ¹ģµĄµÄŌ×Ó | |
| D£® | µē×ÓÅŲ¼Ź½ĪŖ1s2µÄŌ×ÓŗĶĶāĪ§µē×ÓÅŲ¼Ź½ĪŖ2s22p6µÄŌ×Ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ś¢Ū¢Ü¢Ž | B£® | ¢Ś¢Ū¢Ü¢Ż | C£® | ¢Ł¢Ś¢Ū¢Ü¢Ż | D£® | ¢Ł¢Ś¢Ū¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¹żŃõ»ÆÄʵē×ÓŹ½£ŗNa${\;}_{•}^{•}$$\underset{\stackrel{••}{O}}{••}$${\;}_{•}^{•}$$\underset{\stackrel{••}{O}}{••}$${\;}_{•}^{•}$Na | |
| B£® | ÖŹ×ÓŹż35”¢ÖŠ×ÓŹż45µÄäåŌ×Ó£ŗ${\;}_{35}^{80}$Br | |
| C£® | ĮņĄė×Ó½į¹¹Ź¾ŅāĶ¼£ŗ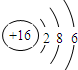 | |
| D£® | ŅŅĻ©µÄ½į¹¹¼ņŹ½£ŗC2H4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com