
 ¹¤ŅµÉĻ£¬æÉŅŌÓÉĻĀĮŠ·“Ó¦ŗĻ³ÉČż¾ŪĒč°·£ŗ
¹¤ŅµÉĻ£¬æÉŅŌÓÉĻĀĮŠ·“Ó¦ŗĻ³ÉČż¾ŪĒč°·£ŗ £®ĘäÖŠµŖŌ×ÓµÄŌӻƷ½Ź½ÓŠsp2”¢sp3£®
£®ĘäÖŠµŖŌ×ÓµÄŌӻƷ½Ź½ÓŠsp2”¢sp3£®·ÖĪö £Ø1£©CrŌ×ÓŗĖĶāµē×ÓŹżĪŖ24£¬øł¾ŻÄÜĮæ×īµĶŌĄķŹéŠ“ŗĖĶāµē×ÓÅŲ¼Ź½£»
£Ø2£©Ō×Ó×ÜŹżĻąĶ¬”¢¼Ūµē×Ó×ÜŹż£Ø»ņµē×Ó×ÜŹż£©ĻąĶ¬µÄĪ¢Į£»„ĪŖµČµē×ÓĢ壻
£Ø3£©CO£ØNH2£©2ÖŠCŌ×ÓÓėŃõŌ×ÓÖ®¼äŠĪ³ÉC=OĖ«¼ü£¬CŌ×ÓÓėNŌ×ÓÖ®¼äŠĪ³É2øöC-Nµ„¼ü£¬NŌ×ÓÓėHŌ×ÓÖ®¼ä¹²ŠĪ³É4øöN-H¼ü£¬µ„¼üŹĒ¦Ņ¼ü£¬Ė«¼üÖŠŗ¬ÓŠ1øö¦Ņ¼üŗĶ1øö¦Š¼ü£»
£Ø4£©»·ÖŠNŌ×ÓŠĪ³É2øö¦Ņ¼ü£¬ŗ¬ÓŠ1¶Ō¹Āµē×Ó¶Ō£¬ŌӻƹģµĄŹżÄæĪŖ3£¬°±»łÖŠNŌ×ÓŠĪ³É3øö¦Ņ¼ü£¬ŗ¬ÓŠ1¶Ō¹Āµē×Ó¶Ō£¬ŌӻƹģµĄŹżÄæĪŖ4£»
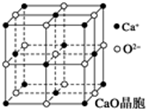
£Ø5£©ŅŌĢåŠÄO2-ŃŠ¾æ£¬ÓėÖ®×ī½üµÄCa2+Ī»ÓŚĆęŠÄ£»µēŗÉŌ½“󔢰ė¾¶Ō½Š”£¬¾§øńÄÜŌ½“ó£¬Ąė×Ó¾§ĢåµÄČŪµćŌ½øߣ¬µēŗÉĪŖÖ÷ŅŖÓ°ĻģŌŅņ£®
½ā“š ½ā£ŗ£Ø1£©CrŌ×ÓŗĖĶāµē×ÓŹżĪŖ24£¬ŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ[Ar]3d54s1£¬¹Ź“š°øĪŖ£ŗ[Ar]3d54s1£»
£Ø2£©Ō×Ó×ÜŹżĻąĶ¬”¢¼Ūµē×Ó×ÜŹż£Ø»ņµē×Ó×ÜŹż£©ĻąĶ¬µÄĪ¢Į£»„ĪŖµČµē×ÓĢ壬ÓėC22-µÄŅ»Öֵȵē×ÓĢåµÄ»ÆѧŹ½ĪŖ£ŗN2”¢CO”¢CN-µČ£¬¹Ź“š°øĪŖ£ŗN2”¢CO”¢CN-µČČĪŠ“Ņ»øö£»
£Ø3£©CO£ØNH2£©2ÖŠCŌ×ÓÓėŃõŌ×ÓÖ®¼äŠĪ³ÉC=OĖ«¼ü£¬CŌ×ÓÓėNŌ×ÓÖ®¼äŠĪ³É2øöC-Nµ„¼ü£¬NŌ×ÓÓėHŌ×ÓÖ®¼ä¹²ŠĪ³É4øöN-H¼ü£¬µ„¼üŹĒ¦Ņ¼ü£¬Ė«¼üÖŠŗ¬ÓŠ1øö¦Ņ¼üŗĶ1øö¦Š¼ü£¬ĖłŅŌ1mol ÄņĖŲ·Ö×Ó[CO£ØNH2£©2]ÖŠŗ¬ÓŠµÄ¦Š¼üÓė¦Ņ¼üµÄŹżÄæÖ®±ČĪŖ1£ŗ7£¬¹Ź“š°øĪŖ£ŗ1£ŗ7£»
£Ø4£©»·ÖŠNŌ×ÓŠĪ³É2øö¦Ņ¼ü£¬ŗ¬ÓŠ1¶Ō¹Āµē×Ó¶Ō£¬ŌӻƹģµĄŹżÄæĪŖ3£¬²ÉČ”sp2Ōӻƣ¬°±»łÖŠNŌ×ÓŠĪ³É3øö¦Ņ¼ü£¬ŗ¬ÓŠ1¶Ō¹Āµē×Ó¶Ō£¬ŌӻƹģµĄŹżÄæĪŖ4£¬²ÉČ”sp3Ōӻƣ¬¹Ź“š°øĪŖ£ŗsp2”¢sp3£»
£Ø5£©ŅŌĢåŠÄO2-ŃŠ¾æ£¬ÓėÖ®×ī½üµÄCa2+Ī»ÓŚĆęŠÄ£¬¹ŹÓėÖ®×ī½üµÄCa2+¹²ÓŠ6£»
CaO¾§ĢåµÄČŪµć±ČNaCl¾§ĢåµÄČŪµćøßµÄÖ÷ŅŖŌŅņŹĒ£ŗCaO¾§ĢåÖŠCa2+”¢O2-µÄµēŗÉŹż±ČNaCl¾§ĢåÖŠNa+”¢Cl-“ó£¬CaO¾§ĢåµÄ¾§øńÄÜ“ó£¬
¹Ź“š°øĪŖ£ŗ6£»CaO¾§ĢåÖŠCa2+”¢O2-µÄµēŗÉŹż±ČNaCl¾§ĢåÖŠNa+”¢Cl-“ó£¬CaO¾§ĢåµÄ¾§øńÄÜ“ó£®
µćĘĄ ±¾ĢāŹĒ¶ŌĪļÖŹ½į¹¹µÄ漲飬Éę¼°ŗĖĶāµē×ÓÅŲ¼”¢»Æѧ¼ü”¢ŌӻƷ½Ź½ÅŠ¶Ļ”¢¾§°ū¼ĘĖć”¢Ąė×Ó¾§ĢåČŪ·Šµć±Č½ĻµČ£¬ŹĒ¶Ōѧɜ×ŪŗĻÄÜĮ¦µÄ漲飬ÄѶČÖŠµČ£®


 ½ņĒŽĢÓż¼ĘĖ抔דŌŖĻµĮŠ“š°ø
½ņĒŽĢÓż¼ĘĖ抔דŌŖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

| “ĪŹż | 1 | 2 | 3 | 4 |
| µĪ¶ØĢå»ż£ØmL£© | 19.22 | 19.18 | 19.80 | 19.20 |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
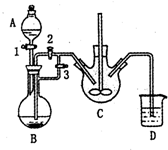 ČéĖįŃĒĢś£Ø[CH3CH£ØOH£©COO]2Fe•3H2O£¬Mr=288£©ŹĒŅ»ÖÖ³£ÓĆµÄ²¹Ģś¼Į£¬æÉĶعżČéĖįÓėĢ¼ĖįŃĒĢś·“Ó¦ÖĘµĆ£ŗ
ČéĖįŃĒĢś£Ø[CH3CH£ØOH£©COO]2Fe•3H2O£¬Mr=288£©ŹĒŅ»ÖÖ³£ÓĆµÄ²¹Ģś¼Į£¬æÉĶعżČéĖįÓėĢ¼ĖįŃĒĢś·“Ó¦ÖĘµĆ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µČĪļÖŹµÄĮæµÄĮņÕōĘųŗĶĮņ¹ĢĢå·Ö±šĶźČ«Č¼ÉÕ£¬ŗóÕ߷ųöČČĮæ¶ą | |
| B£® | ÓÉH+£Øaq£©+OH-£Øaq£©=H2O£Øl£©”÷H=-57.3kJ•mol-1æÉÖŖ£¬Čō½«ŗ¬1 mol CH3COOHµÄĻ”ČÜŅŗÓėŗ¬1 mol NaOHµÄĻ”ČÜŅŗ»ģŗĻ£¬·Å³öµÄČČĮæŠ”ÓŚ57.3kJ | |
| C£® | 300”ę”¢30MPaĻĀ£¬½«0.5molN2£Øg£©ŗĶ1.5mol H2£Øg£©ÖĆÓŚĆܱÕČŻĘ÷ÖŠ³ä·Ö·“Ӧɜ³ÉNH3£Øg£©£¬·ÅČČ19.3kJ£¬ĘäČČ»Æѧ·½³ĢŹ½ĪŖ£ŗN2£Øg£©+3H2£Øg£©?2NH3£Øg£©”÷H=-38.6kJ•mol-1 | |
| D£® | ÓÉC£ØŹÆÄ«£©=C£Ø½šøÕŹÆ£©”÷H=+1.90 kJ•mol-1æÉÖŖ£¬½šøÕŹÆ±ČŹÆÄ«ĪČ¶Ø |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £¬ĒāŌŖĖŲŗ¬Įæ×īøßµÄĢžµÄ·Ö×ÓŹ½CH4£®
£¬ĒāŌŖĖŲŗ¬Įæ×īøßµÄĢžµÄ·Ö×ÓŹ½CH4£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com