
��������![]() �ֳ�˫��ˮ������һ�ֶ�Ԫ���ᣬ��̼������Ի��������ֽ⣬��˫��ˮ��������������������������Ϊ��ɫ����������������ҵ���ŷdz��㷺����;����ҽҩ�������º͵�����ɱ��������ҵ������Ư��ë��˿����ë�ĺ����ﵰ���ʵ�֯�ʵ���б��㷺�����������ȡ�
�ֳ�˫��ˮ������һ�ֶ�Ԫ���ᣬ��̼������Ի��������ֽ⣬��˫��ˮ��������������������������Ϊ��ɫ����������������ҵ���ŷdz��㷺����;����ҽҩ�������º͵�����ɱ��������ҵ������Ư��ë��˿����ë�ĺ����ﵰ���ʵ�֯�ʵ���б��㷺�����������ȡ�
![]() (1)�
(1)д��![]() �ĵ���ʽ ��˫��ˮ����Ϊ��ɫ��������ԭ��
�ĵ���ʽ ��˫��ˮ����Ϊ��ɫ��������ԭ��
![]() ��
��
![]() (2) ����������̺�����Ӧ�Ļ�ѧ����ʽ ��Ӧ�ж�����
(2) ����������̺�����Ӧ�Ļ�ѧ����ʽ ��Ӧ�ж�����
![]() �̵����� ��
�̵����� ��
![]() (3)���õ��ͻ���ɫ��λ(
(3)���õ��ͻ���ɫ��λ(![]() )������(PbS)����˫��ˮ�������ָֻ�ԭò���йط�Ӧ�Ļ�ѧ����ʽΪ ��
)������(PbS)����˫��ˮ�������ָֻ�ԭò���йط�Ӧ�Ļ�ѧ����ʽΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 17Cv |
| 200�� |
| 17Cv |
| 200�� |
| ʵ������ | ʵ�鷽�� | ʵ������ |
| ��֤������ | ȡ�����⻯�ص�����Һ��ϡ�������Թ��У��������������Һ������ȡ��������������Һ���Թ��У��������������Һ���� | ��Һ����ɫ ��Һ����ɫ ������ ��������ɫ��������Һ����� ��������ɫ��������Һ����� �� |
| ��֤�Ȳ��ȶ��� | ȡ��������������Һ���Թ��У� ������ ������ �ô����ǵ�ľ������ �ô����ǵ�ľ������ �� |
�������ݣ������ǵ�ľ����ȼ�� |
| �¶ȣ��棩 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| ͭƽ���ܽ����� ����10-3mol?L-1?min-1�� |
7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʦ����2009�������ѧ�����п���(��ѧ) ���ͣ�022
���������ֳ�˫��ˮ������һ�ֱ�̼�ỹ���Ķ�Ԫ���ᣬ�����Ȼ���������¼����ȶ����ֽ�����H2O��O2������������Ϊ��ɫ���������о�������������ͼ��ʾ�Ŀռ�����ṹ���ݴ˻ش��������⣺
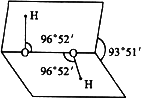
(1)д��˫��ˮ�ĵ���ʽ��________������________����(����ԡ��Ǽ��ԡ�)�����뷽��ʽ________��
(2)˫��ˮ��ʹ���Ը��������Һ��ɫ��������˫��ˮ��________��(�������������ԭ�����ᡱ)����Ӧ�����ӷ���ʽ��________��
(3)��H2O2��Һ���뺬�з�̪��NaOH��Һ�У���ɫ��ʧ������Ϊ��ɫ��ʧ��ԭ����H2O2���е�________��(�������������ԭ�����ᡱ)�������ʵ��֤����Ĺ۵㣺________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)д��˫��ˮ�ĵ���ʽ________________________�����뷽��ʽ______________________��
(2)˫��ˮ��ʹ�������������Һ��ɫ��������˫��ˮ��_____________��(�����������ԭ�����ᡱ�������ͬ)��˫��ˮ��ʹ����̪��NaOH��Һ��ɫ��������˫��ˮ��__________�ԡ�
(3)����Ϊ˫��ˮ����Ϊ��ɫ����������Ҫԭ���ǣ�___________________________________��
(4)��֪�������������Ķ�����̼���岻��Ӧ�������Ժͳ�ʪ�Ķ�����̼���巢����Ӧ�����û�ѧ����ʽ��ʾ�������ƺͳ�ʪ�Ķ�����̼���巢����Ӧ�Ĺ��̣�____________________��
(5)����H2O2��������д������Ba(OH)2�����γ����εĻ�ѧ����ʽ��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)��������![]() �ֳ�˫��ˮ������һ�ֶ�Ԫ���ᣬ��̼������Ի��������ֽ⣬��˫��ˮ��������������������������Ϊ��ɫ����������������ҵ���ŷdz��㷺����;����ҽҩ�������º͵�����ɱ��������ҵ������Ư��ë��˿����ë�ĺ����ﵰ���ʵ�֯�ʵ���б��㷺�����������ȡ�
�ֳ�˫��ˮ������һ�ֶ�Ԫ���ᣬ��̼������Ի��������ֽ⣬��˫��ˮ��������������������������Ϊ��ɫ����������������ҵ���ŷdz��㷺����;����ҽҩ�������º͵�����ɱ��������ҵ������Ư��ë��˿����ë�ĺ����ﵰ���ʵ�֯�ʵ���б��㷺�����������ȡ�
(1)д��![]() �ĵ���ʽ ��˫��ˮ����Ϊ��ɫ��������ԭ����
�ĵ���ʽ ��˫��ˮ����Ϊ��ɫ��������ԭ����
(2)����������̺�����Ӧ�Ļ�ѧ����ʽ ��Ӧ�ж������̵����� ��
(3)���õ��ͻ���ɫ��λ(![]() )������( PbS )����˫��ˮ�������ָֻ�ԭò���йط�Ӧ�Ļ�ѧ����ʽΪ ��
)������( PbS )����˫��ˮ�������ָֻ�ԭò���йط�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com