
| 92g/mol |
| 73.6g/mol |
| 92g/mol |
| 73.6g/mol |
| 0.25 |
| 1 |
| a-c |
| a |
| b-3(a-c) |
| 1 |
| a-c |
| a |


 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʱ��/min | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 10 |
| �����¶�/�� | 25 | 70 | 80 | 100 | 130 | 250 | 300 | 350 | 400 | 450 |
| ��������/g | 42.2 | 42.2 | 40.1 | 36.8 | 36.8 | 36.8 | 36.8 | 36.8 | 32.0 | 32.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ҵ���Ի�����Ϊԭ�ϣ����ýӴ����������ᣮ��ش��������⣺
��ҵ���Ի�����Ϊԭ�ϣ����ýӴ����������ᣮ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
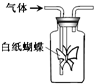 ��ͼ����ƿ������ֽ�۳ɵ�ֽ������������һ����Һ��ͨ��ij�������ʵ������Ԥ���ֽ������ɫ�仯��һ�µ��ǣ�������
��ͼ����ƿ������ֽ�۳ɵ�ֽ������������һ����Һ��ͨ��ij�������ʵ������Ԥ���ֽ������ɫ�仯��һ�µ��ǣ�������| A | B | C | D | |
| ����Һ | ��̪ | ��ɫ���� | ��ɫʯ�� | �ữ��KI-���� |
| ͨ������� | NH3 | Cl2 | SO2 | O2 |
| Ԥ�����ɫ�仯 | ��Ϊ��ɫ | �ȳȺ���ɫ | �Ⱥ����ɫ | ��Ϊ��ɫ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���������ϳɰ������ȷ�Ӧ���·��ϳɰ��Ƿ��ȷ�Ӧ |
| B���·��ϳɰ�����Ҫ�ڸ��������£��ɽ�Լ������Դ�����з�չǰ�� |
| C���·��ϳɰ����ڳ����½�������Ϊ����Ҫ���ѻ�ѧ�� |
| D���´��������˷�Ӧ����Ҫ��������ʹƽ�������ƶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
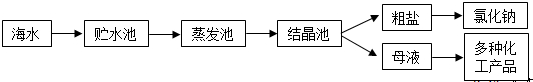
| ���� | Na+ | Mg2+ | Cl- | SO42- |
| Ũ��/��g?L-1�� | 63.7 | 28.8 | 144.6 | 46.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��HCl |
| B��K2SO4 |
| C��FeCl3 |
| D��NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
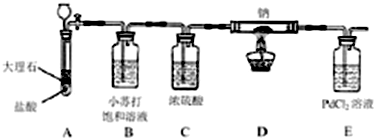
| ��� | ʵ����� | ʵ������ | ���� |
| 1 | ����Ӧ��ȫ��װ��D�еIJ������������ˮ�ܽ⣮ | �к�ɫ������ | |
| 2 | ȡ��������Һ�ϲ���Һ�������BaCl2��Һ | ��������Na2CO3���� | |
| 3 | - | ��������CO���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com