
[��ѧ��ѡ��2��ѧ�뼼��]��15�֣�
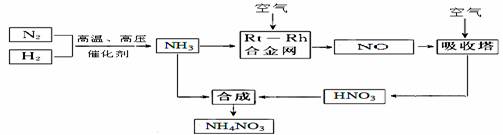 ��������ִ�ũҵ���������ж�ռ����Ҫ��λ����ͼ��������Ȼ���������ϳ�����淋ļ�Ҫ�����������̣�
��������ִ�ũҵ���������ж�ռ����Ҫ��λ����ͼ��������Ȼ���������ϳ�����淋ļ�Ҫ�����������̣�
�ش��������⣺
��1��N2�ĵ���ʽ ���ϳɰ��ķ�Ӧ�У�������1g���ų�����a KJ,д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��2�����������Ļ�ѧ����ʽ�� ��
�Դӻ�ѧ��Ӧ���ʺͻ�ѧƽ��ԭ�������¶ȡ�ѹǿ�Ժϳɰ���Ӧ��Ӱ��
��
��3��������NH3�Ĺܵ�ij������й©�����ļ���
��
��4��ũҵ�����ϳ��ڡ�����ʹ�û�������泥�����������ữ��ԭ���ǣ������ӷ���ʽ�ش� ��
��5��25��ʱ����x mol NH4NO3����һ����ˮ�У������Һ�еμ�y L��ˮ����Һ�����ԣ���μӰ�ˮ������ˮ�ĵ���ƽ�⽫ ������� �������������ƶ������μӰ�ˮ�����ʵ���Ũ��Ϊ ��25��ʱ��Kb(NH3��H2O)=2.0 �� 10-5 mol��L-1����
[��ѧ��ѡ��2��ѧ�뼼��]
��1��  ��2�֣�N2(g)+3H2 (g)
��2�֣�N2(g)+3H2 (g)  2NH3 (g) ��H=-34a kJ/mol ��2�֣�
2NH3 (g) ��H=-34a kJ/mol ��2�֣�
��2��4NH3 + 5O2 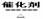 4 NO +6H2O ��2�֣�
4 NO +6H2O ��2�֣�
�ϳɰ�����Ӧ���ȣ��������������ת���ʣ�����Ӧ���ʹ������ʺϳɰ����ø��£�400��-500�棩������Ӧ�����С������ѹǿ���������ת���ʣ������ƶ������ʲ��ø�ѹ��10 MPa -30MPa������3�֣�
��3������ʪ�ĺ�ɫʯ����ֽ��⣬��ֽ������λ�ü��ǰ���й©����������������Ҳ�ɣ���2�֣�
��4��NH4++ H2O  NH3 ��H2O +H+��2�֣�
NH3 ��H2O +H+��2�֣�
��5������1�֣� x/200y mol��L��1��2�֣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ������������ԭ����Ͳ���������(����)
| ѡ�� | ʵ����� | ʵ������ | ԭ����� |
| A | ��һƬ�ϱ�����Ƭ���ھƾ��ƻ��������� | ��Ƭ�����ۻ���Һ��״ | �����������Ĥ�������۵�ܸߣ��谭���������ĽӴ���ͬʱ�����۵�ϵͣ��ۻ��� |
| B | ���ȱ������þ������Ƭ(����ȥ����Ĥ)�ֱ����װ�е�Ũ�ȡ�������������֧�Թ��� | ���������ݣ�þ������������ݸ��� | þ�Ľ����Ա����Ľ�����ǿ��þ����ʧȥ���ӣ����Է�Ӧ���� |
| C | �ڴ�ĥ������Ƭ���ȵ�һ�α���ʳ��ˮ���ٵ�һ�η�̪ | Һ�ε���Ȧ�ȳ��ֺ�ɫ | ��Ƭ��������̼Ԫ�أ�����̼��ʳ��ˮ�γ�ԭ��أ���Һ��Ȧ����������ʴ������c(OH��)�� ����Һ�ʼ��� |
| D | ȡ�������ۺ�ϡ���ᣬ���ȼ����ӣ���ȴ���������������ͭ������������ | δ��ש��ɫ�������� | ����ˮ���û������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�о���ѧϰС���ij������������(FeSO4��xH2O)�ȷֽ��о�����С��ͬѧ��ȡa g��������������Ʒ��ͼ1���¼��ȣ�ʹ����ȫ�ֽ⣬�����ò������̽������ͨ������װ��B�������x��ֵ��

(1)װ��B������ͭ��ĩ��������������12.6 g��˵����������ˮ��װ��C�и��������Һ��ɫ��˵�������л���________________��
(2)ʵ����Ҫ����ͨ�뵪������������x��_________ (�ƫ����ƫС�����䡱)��
(3)��������������ȫ�ֽ��װ��A�л���������ɫ����Fe2O3��
(4)�������Ϸ����ó����������ֽ������һ����SO3��д��FeSO4�ֽ�Ļ�ѧ����ʽ________________��
(5)װ��D���θ���ܵ�����________________��
(6)ij�о�������SDTQ600�ȷ����Ƕ�������������(FeSO4��xH2O)�����ȷֽ⣬���������ݣ����Ƴɹ����������ֽ��¶ȵĹ�ϵͼ��ͼ2������ͼ2���й����ݣ��ɼ����FeSO4��xH2O�е�x��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���ܴﵽʵ��Ŀ�ĵ���
| ѡ�� | ʵ����� | ʵ��Ŀ�� |
| A | �����£��ⶨ��Ũ�ȵ�CH3COOH��Һ�������pH | �Ƚ������������ǿ�� |
| B | CH3CH2Br �м���NaOH��Һ���ȳ�ַ�Ӧ��ȡ�ϲ�ˮ��Һ����AgNO3��Һ | ����CH3CH2Br�е���ԭ�� |
| C | ��25ml��ˮ�еμ�4��5�α���FeCl3��Һ���������к��ɫ�������� | �Ʊ�Fe(OH)3���� |
| D | ��ij��Һ���ȼ���HNO3��Һ���ټ���Ba(NO3)2��Һ | ������Һ���Ƿ����SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ��������6ƿʧȥ��ǩ�İ�ɫ���壺���������þ���Ȼ��������������������ơ��Ȼ��ء�������ˮ���Թܺͽ�ͷ�ι��⣬�������κ��Լ���������ijѧ��ͨ������ʵ�鲽�輴�ɼ������ǡ�����д���пհף�
(1)��ȡ����������6֧�Թ��У��ֱ������������ˮ����һ֧�Թ��е����������5֧���Բ�ͬ�����Թ��е�������
________________________________________________________________________��
�ݴ�����������һ��������__________��
(2)�ֱ���ʣ5����Һ���α��ΪA��B��C��D��E��Ȼ�����������ϡ��۲쵽Cû�г����κ�����D�ֱ��A��B��E���ʱ�������˰�ɫ������B��E���ʱ���а�ɫ����������������ɫ����ų����ݴ˿��ƶϳ���
��A��C��D�������ʵĻ�ѧʽ������________________________________________��
��B��E��������һ�ֿ���A��Ӧ����������A��Ӧ�����ӷ���ʽΪ________________________________________________________________________��
�����������ʱ��������ȷ��B��E�ɷֵ�ʵ����������_____________________
___________________________________________________��
(3)������������ˮ����ˮ�ĵ��룬����Һ�����Ե����ʵĻ�ѧʽΪ________������Һ�����Ե�ԭ����___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����仯�������ճ�������Ӧ�ù㷺�����������ʵ��ش����⣺
(1)�����ڳ�ʪ�Ŀ����з���������ʴ��������ӦʽΪ________________________��
(2)�����к���һ����̼������X(Fe3C)��X�������Ŀ����и������գ������д��ԵĹ���Y��Y�����ڹ������ᣬ�γɻ���ɫ����Һ��д��Y�����ᷴӦ�Ļ�ѧ����ʽ��_____________________________________________________________________��
(3)ij���������к��д���CuS���������Ļ������ҵ���Ը÷���Ϊԭ������CuCl2·2H2O�Ĺ����������£�
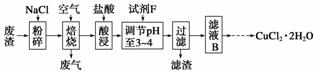
��֪��a.Fe(OH)2��Fe(OH)3��Cu(OH)2������ȫʱ��pHֵ�ֱ�Ϊ9.7��3.2��6.4��
b����������ʱCuS��������Ҫ��ӦΪCuS��NaCl��O2����CuCl2��Na2SO4(δ��ƽ)��
�Իش��������⣺
���Լ�FӦѡ��________(����)��
A��Cl2 B��NaClO C��HNO3 D��Ũ����
������_______________________________________________________________��
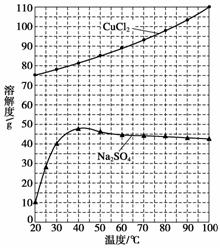
��Ϊ�˻��CuCl2·2H2O���壬����ҺB���еIJ���������Ũ�������ȹ��ˣ���Һ����ȴ�ᾧ�����˵õ���Ʒ�������й����ʵ��ܽ������(��ͼ)�������ȹ��ˡ��õ��Ĺ�����____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ��NaCl��Na2CO3·10H2O��NaHCO3�Ļ���ijͬѧ�����ͼʵ�飬ͨ��������Ӧǰ��C��Dװ�������ı仯���ⶨ�û�����и���ֵ�����������
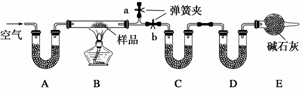
(1)����ǰͨ�������Ŀ����_______________________________________________��
��������Ϊ_______________________________________________________________
________________________________________________________________________��
(2)װ��A��C��D��ʢ�ŵ��Լ��ֱ�Ϊ��A______________��C______________��D________________��
(3)����Aװ�û���ʢ��NaOH��Һ��ϴ��ƿ�����õ�NaCl�ĺ�����________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족����ͬ)����B�з�Ӧ���Ҳ���ˮ������������ⶨ�����NaHCO3�ĺ�����________������ȥEװ�ã�����Na2CO3·10H2O �ĺ�����________��
(4)����Ʒ����Ϊw g����Ӧ��C��D���ӵ������ֱ�Ϊm1 g��m2 g���ɴ˿�֪�������NaHCO3����������Ϊ_______________________________________________________
(�ú�w��m1��m2�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ����٤����������ֵ������˵����ȷ����
A�������£�32g SO2����NA����ԭ��
B��0.1mol•L-1�İ�ˮ����0.1NA��OH�D
C�����³�ѹ�£�22.4LCCl4���и�NA��CCl4����
D��1mol��������������Ӧ��ת��2NA������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com