


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
|
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
 £»
£» ”£
”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
 £© ”£
£© ”£ 1000mLČŻĮæĘæ D ²£Į§°ō
1000mLČŻĮæĘæ D ²£Į§°ō ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
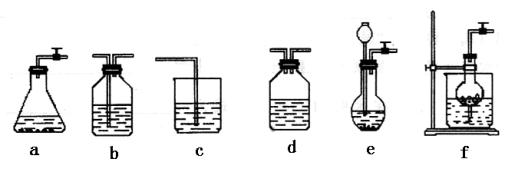
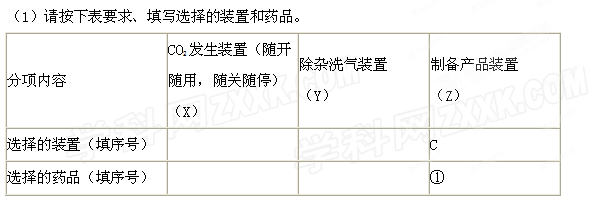
 ¹żĮæCO2ŗó£¬Z×°ÖĆÄŚµÄČÜŅŗÖŠĄė×ÓÅØ¶Č“óŠ”Ė³ŠņĪŖ””””””””””””””””””””””””””””””””””””””£»ČōŅŖ±£Ö¤ŅŅ×°ÖĆÖŠ²»Īö³ö¾§Ģå£Ø²»æ¼ĀĒ¹ż±„ŗĶČÜŅŗĪŹĢā£©£¬NaOHČÜŅŗ×ī“óÅØ¶Č²»Äܳ¬¹ż””””””””””%£ØÖŹĮæ·ÖŹż£©”£
¹żĮæCO2ŗó£¬Z×°ÖĆÄŚµÄČÜŅŗÖŠĄė×ÓÅØ¶Č“óŠ”Ė³ŠņĪŖ””””””””””””””””””””””””””””””””””””””£»ČōŅŖ±£Ö¤ŅŅ×°ÖĆÖŠ²»Īö³ö¾§Ģå£Ø²»æ¼ĀĒ¹ż±„ŗĶČÜŅŗĪŹĢā£©£¬NaOHČÜŅŗ×ī“óÅØ¶Č²»Äܳ¬¹ż””””””””””%£ØÖŹĮæ·ÖŹż£©”£| »ÆѧŹ½ | Na2CO3 | NaHCO3 | NaOH | NaCl | Na2SO4 |
| Čܽā¶Č£Øg/100gH2O£© | 21.3 | 9.60 | 107 | 35.8 | 19.4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
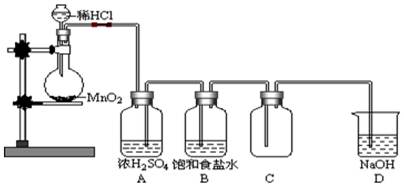
 Źö×°ÖĆĶ¼ÖŠµÄ“ķĪó£ØÓŠ¼ø“¦“š¼ø“¦£©
Źö×°ÖĆĶ¼ÖŠµÄ“ķĪó£ØÓŠ¼ø“¦“š¼ø“¦£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
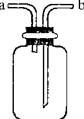
| A£®øÉŌļCO2£ŗĘæÄŚ·ÅŅ»¶ØĢå»żÅØĮņĖį£¬ÓÉa½ųĘų |
| B£®ÓĆÓŚŹÕ¼ÆO2£ŗĘæÄŚ³äĀśĖ®£¬ÓÉb½ųĘų |
| C£®Ģį¹©ÉŁĮæCO£ŗĘæÄŚ³äĀśCO£¬b½Ó½ųĖ®¹Ü |
| D£®ÓĆ×öH2SŗĶSO2·“Ó¦µÄ×°ÖĆ£ŗÓÉaĶØČėH2S£¬bĶØČėSO2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
 ÖĘČÜŅŗŹ±×īŗóĮ½øö²½Öč£¬°“Ęä²Ł×÷¶ØČŻ£¬¶ŌĖłÅäČÜŅŗÅضČÓŠŗĪÓ°Ļģ£æ ”””” £ØĢī”°Ę«øß”±”¢”±Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±£©£»
ÖĘČÜŅŗŹ±×īŗóĮ½øö²½Öč£¬°“Ęä²Ł×÷¶ØČŻ£¬¶ŌĖłÅäČÜŅŗÅضČÓŠŗĪÓ°Ļģ£æ ”””” £ØĢī”°Ę«øß”±”¢”±Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±£©£»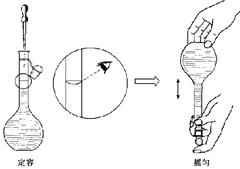
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com