

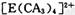 �����ӣ����д��ڵĻ�ѧ�������� ______ (����ţ���
�����ӣ����д��ڵĻ�ѧ�������� ______ (����ţ���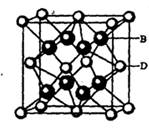
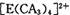 ���жԳƵĿռ乹�ͣ��ҵ�
���жԳƵĿռ乹�ͣ��ҵ�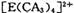 �е�����CA3������Cl-ȡ��ʱ���ܵõ����ֲ�ͬ�ṹ�IJ����
�е�����CA3������Cl-ȡ��ʱ���ܵõ����ֲ�ͬ�ṹ�IJ����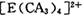 �Ŀռ乹��Ϊ ____________ (�����)��
�Ŀռ乹��Ϊ ____________ (�����)��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������Ԫ����ǽ���Ԫ��֮�䶼�γ����Ӽ� |
| B���γ����Ӽ����������ǽ����������������� |
| C���������Ӽ��Ļ������У�Ҳ���ܻ����й��ۼ� |
| D���ǽ���Ԫ���γɵĻ�����һ�����������Ӽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
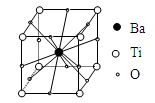
| A��BaTi8O12 | B��BaTi4O6 |
| C��BaTi2O4 | D��BaTiO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
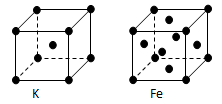
�鿴�𰸺ͽ���>>
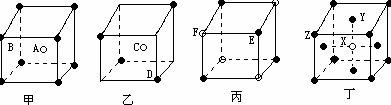
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Ȼ�菉����У�ÿ1��Cs+������8��Cs+�Ⱦ������ |
| B�����ʯ��״�ṹ�У��ɹ��ۼ����ɵ�̼ԭ�ӻ��У���С�Ļ�����4��̼ԭ�� |
| C��������������ֶѻ�ģ���У��������ܶѻ��������������ܶѻ��Ŀռ���������� |
| D��PCl3����BCl3����������ԭ�ӵ�����㶼�ﵽ8�����ȶ��ṹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
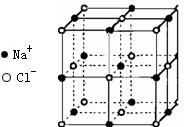
 (4)������,Fe���Ӽ��������Ϊ cm
(4)������,Fe���Ӽ��������Ϊ cm�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��KCl | B��NaBr | C��LiI | D��KF |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com