
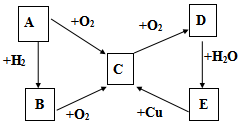
 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ����� ת����ϵ�У�A��������������Ӧ���ﶼ���Լ�����������Ӧ��˵��A�е�Ԫ���DZ��Ԫ�أ����ڷǽ�����A+O2��C��C+O2��D��D+H2O��E��֤��EΪ�ᣬB+O2��C��C+O2��D��E+Cu��C��˵��E��ǿ�����Ե��ᣬ
��1����AΪ���嵥�ʣ����ж�ΪS����ת����ϵ��֪BΪH2S��CΪSO2��DΪSO3��EΪH2SO4��
��2����AΪ���嵥�ʣ����ɵ����ͭ��Ӧ���ƶ�ΪN2����BΪNH3��CΪNO��DΪNO2��EΪHNO3��
��� �⣺ת����ϵ�У�A��������������Ӧ���ﶼ���Լ�����������Ӧ��˵��A�е�Ԫ���DZ��Ԫ�أ����ڷǽ�����A+O2��C��C+O2��D��D+H2O��E��֤��EΪ�ᣬB+O2��C��C+O2��D��E+Cu��C��˵��E��ǿ�����Ե��ᣬ
��1����AΪ���嵥�ʣ����ж�ΪS����ת����ϵ��֪BΪH2S��CΪSO2��DΪSO3��EΪH2SO4��
�������Ϸ�����֪D�Ļ�ѧʽΪSO3���ʴ�Ϊ��SO3��
��E��C�Ļ�ѧ����ʽ��Ũ�����ͭ��Ӧ��������ͭ�����������ˮ����Ӧ�Ļ�ѧ����ʽΪ2H2SO4��Ũ��+Cu $\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
�ʴ�Ϊ��2H2SO4��Ũ��+Cu $\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
��Cͨ������NaOH�е����ӷ�Ӧ����ʽΪSO2+OH-=HSO3-��
�ʴ�Ϊ��SO2+OH-=HSO3-��
��2����AΪ���嵥�ʣ����ɵ����ͭ��Ӧ���ƶ�ΪN2����BΪNH3��CΪNO��DΪNO2��EΪHNO3��
��B��C�Ļ�ѧ����ʽΪ4NH3+5O2$\frac{\underline{\;\;��\;\;}}{\;}$ 4NO+6H2O���ʴ�Ϊ��4NH3+5O2$\frac{\underline{\;\;��\;\;}}{\;}$ 4NO+6H2O��
��E��C�����ӷ���ʽΪ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O���ʴ�Ϊ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
���� ���⿼��������ƶϣ�Ϊ��Ƶ���㣬����Ԫ�ػ�����֪ʶ�����ʵ����ʡ��ת��Ϊ���Ĺؼ������ط������ƶ������Ŀ��飬ע��N��S�Ļ��������ʣ���Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����̿��SO2��Na2O2����ʹƷ����Һ��ɫ����ԭ����ͬ | |
| B�� | �ǽ��������ﲻһ���������������������������Ǽ��������� | |
| C�� | ͬ��ͬѹ�£�������ͬ���������ۣ��ֱ���������ϡ�����ϡ���ᷴӦ����������������ͬ | |
| D�� | ��SO2����ͨ��BaCl2��Һ��������δ���������ɣ�����ͨ��NO2���г������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ž�����Ʒ�����������е�Ӧ�� | |
| B�� | ���������������ڴ�����Ի����ʳƷ | |
| C�� | ���Ի��ٳ�ʹ���˺���ʳƷ���Ӽ���ʳ�� | |
| D�� | ��������������ҩ������θ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
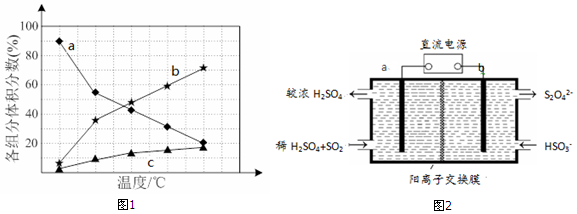
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ճ�������ʹ�õ�Ӳ�Ҷ��Ǵ��𡢰�������� | |
| B�� | �ܻ����������к�����������ʳƷ���Ӽ� | |
| C�� | SO2������Ư��ֽ�ţ����Ư���ճ�������ʳ����ͷ | |
| D�� | ��ҩ�����֡���ʵ��û�п�ѧ���ݵģ�ֻҪ��Ч������Ҳ����ν |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com