
| A�� | ����£�ȼ��1mol S�ų�������Ϊ297.23 kJ | |
| B�� | S �� g ��+O2 �� g ��=SO2 �� g �� �ų�����������297.23 kJ | |
| C�� | S �� g ��+O2 �� g ��=SO2 �� g �� �ų�������С��297.23 kJ | |
| D�� | �γ�1 mol SO2 �Ļ�ѧ�����ͷŵ����������ڶ��� 1 molS �� s ���� 1mol O2 �� g ���Ļ�ѧ�������յ������� |
���� ��S��s��+O2��g���TSO2��g����H=-297.23kJ/mol����֪�÷�ӦΪ���ȷ�Ӧ��S��s��=S��g��Ϊ���ȹ��̣��ɸ�˹���ɿ�֪��S��g��+O2��g���TSO2��g����H��-297.23kJ/mol���Դ������
��� �⣺��S��s��+O2��g���TSO2��g����H=-297.23kJ/mol��
A��1molS��״̬��֪�����������壬Ҳ�����ǹ��壬��A����
B��S��s��=S��g��Ϊ���ȹ��̣��ɸ�˹���ɿ�֪��S��g��+O2��g���TSO2��g����H��-297.23kJ/mol����ų�����������297.23 kJ����B��ȷ��
C��S��s��=S��g��Ϊ���ȹ��̣��ɸ�˹���ɿ�֪��S��g��+O2��g���TSO2��g����H��-297.23kJ/mol����ų�����������297.23 kJ����C����
D���÷�ӦΪ���ȷ�Ӧ�����γ�1 mol SO2�Ļ�ѧ�����ͷŵ����������ڶ���1 mol S��s����1 mol O2��g���Ļ�ѧ�������յ�����������D��ȷ��
��ѡAC��
���� ���⿼��ȼ���ȵ��Ȼ�ѧ��Ӧ����ʽ����ȷȼ�յĸ����Ӧ������ܡ���˹���ɵĹ�ϵ���ɽ����Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ƽ��ʱ��������I2�����ʵ������ | |
| B�� | ƽ��ʱ��������I2��Ũ����� | |
| C�� | ƽ��ʱ�������ڻ�������еİٷֺ�����A��������B���� | |
| D�� | ƽ��ʱHI�ķֽ��ʣ���A=��B |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
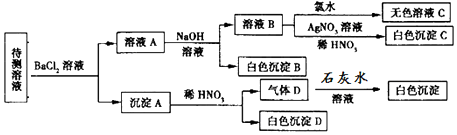
| A�� | SO42-��SO32-���ٺ���һ�� | |
| B�� | ����B�Ļ�ѧʽΪBaCO3 | |
| C�� | �϶����ڵ���������CO32-��HCO3-��Cl- | |
| D�� | �϶�û�е�������Br- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���е�������Ϊa+b | B�� | ���еĵ�����Ϊa-n | ||
| C�� | ������Ϊa+b+n | D�� | 1mol��ԭ�ӵ�����ԼΪbg |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
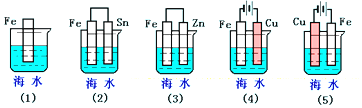
| A�� | ��5����2����1����3����4�� | B�� | ��2����5����3����4����1�� | C�� | ��5����3����4����1����2�� | D�� | ��1����5����3����4����2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
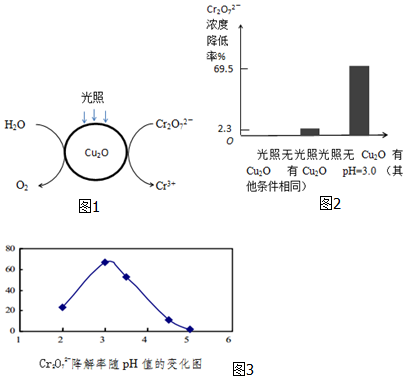
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com