
��̽�������������ʻ���ɵ�ʵ���У�ʵ��������ʵ����۾���ȷ���ǣ� ��
| ѡ�� | ʵ������ | ʵ������ | ʵ����� |
| A | ������ˮ���������в����裬�ټ���Ũ���ᣬѸ�ٽ��� | ������ڣ�������ͣ��д̼�����ζ�������� | ֻ������Ũ�������ˮ�� |
| B | ��ͭƬ����Ũ������ | ����������ɫ���壬��Һ��Ϊ����ɫ | ֻ������Ũ�����ǿ������ |
| C | �����������������Һ��ַ�Ӧ��������ϡ�����ữ���ټ�����������Һ | ���ɵ���ɫ���� | �������к�����Ԫ�� |
| D | ����ˮ���뱽�в������ | ��ˮ��ɫ | �����巢����ȡ����Ӧ |
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����



| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 �о���ѧϰС��Ϊ̽��Cu��ŨH2SO4��Ӧ�������SO2�����ʣ��������ʵ��װ�ã�
�о���ѧϰС��Ϊ̽��Cu��ŨH2SO4��Ӧ�������SO2�����ʣ��������ʵ��װ�ã�
| ||
| ||

| ���� | �ܽ�ȣ�g/100ˮ�� | ���� | �ܽ�ȣ�g/100ˮ�� |
| Ca��OH��2 | 0.173 | Ba��OH��2 | 3.89 |
| CaCO3 | 0.0013 | BaSO3 | 0.016 |
| Ca��HCO3��2 | 16.60 |
| ʵ�鷽�� | ���� |
| 1ȡ������Һ���Թ��У���������������Һ | |
| 2ȡ������Һ���Թ��У�����ϡ���ᣬ���ȣ���ʪ�����ɫʯ����ֽ�������ɵ����壮 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008-2009ѧ��ȱ������е�һѧ�ڸ������п��ԡ���ѧ�Ծ� ���ͣ�013
��̽�������������ʻ���ɵ�ʵ���У�ʵ��������ʵ����۾���ȷ����________
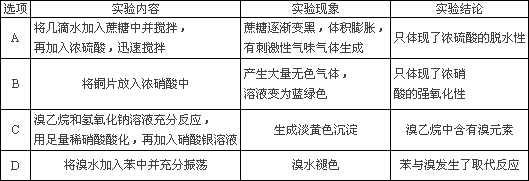
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��̽�������������ʻ���ɵ�ʵ���У�ʵ��������ʵ����۾���ȷ���ǣ� ��
| ѡ�� | ʵ������ | ʵ������ | ʵ����� |
| A | ������ˮ���������в����裬�ټ���Ũ���ᣬѸ�ٽ��� | ������ڣ�������ͣ��γ����ɶ������ | ֻ������Ũ�������ˮ�� |
| B | ��ͭƬ����Ũ������ | ����������ɫ���壬��Һ��Ϊ����ɫ | ֻ����Ũ�����ǿ������ |
| C | ���������������Һ��ַ�Ӧ��������ϡ�����ữ���ټ�����������Һ | ���ɵ���ɫ���� | �������к�����Ԫ�� |
| D | ����ˮ���뱽�в������ | ��ˮ��ɫ | �����巢����ȡ����Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com