
��14�֣�����A��B��C��D�������ʣ�B��D����ɫ��Ӧ��Ϊ��ɫ��C��һ����ʽ�Σ���C����BaCl2��Һ�в���������İ�ɫ�������ɣ�D�����ڸ��������������ʧȥ�ᾧˮ��ɰ�ɫ��ĩ����A��B��C��D��������ʵ�飬ʵ����̺ͼ�¼����ͼ��ʾ������������ȥ������ش�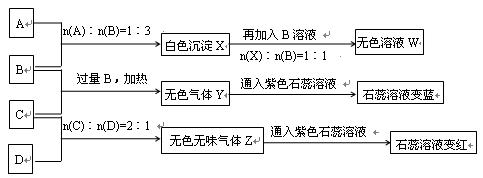
��1��X��B��Ӧ�����ӷ���ʽΪ����������������������������������������������������
��2��D��ҺpHֵ����7��ԭ���ǣ������ӷ���ʽ��ʾ����������������������������������
��3��д��C�����B��Ӧ�����ȣ������ӷ���ʽ����__________________��������������
��4����B��C��ϡ��Һ��Ϻ����ȣ���Һ�����ԣ������Һ������Ũ�ȴӴ�С��˳��_____________________.
��5��Y��ͬ�����ͬ�������з����ȶ�����ǿ�� ����Ҳ�Ƿе���ߵģ� �������ж�������"��"��"��"��
��6����������������B��ϡ��Һ�зֱ��������ϡ���ᡢϡ���ᡢŨ���ᣬ������1molH2Oʱ�ķ�Ӧ�ȷֱ�Ϊ��H1 ����H2����H3 ���������ɴ�С����Ϊ��___________________.
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com