
|
����NH4I����һ�����ΪVL������ܱ������У�������һ���¶Ⱥ����·������з�Ӧ��NH4I(s) | |
| [����] | |
A�� |
ƽ��ʱ����Ϊ2.5 mol |
B�� |
ƽ��ʱHI�ķֽ���Ϊ20�� |
C�� |
����ʼʱ�������NH4I��������һ������ƽ��ʱ���干Ϊ10 mol |
D�� |
������������������������С��V/2 L�����´ﵽƽ��ʱH2��Ũ����ԭƽ���2�� |
 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����NH4I����һ�����ΪVL������ܱ������У�������һ���¶Ⱥ����·������з�Ӧ��NH4I(s) NH3(g)��HI(g)��2HI(g)
NH3(g)��HI(g)��2HI(g) H2(g)��I2(g)��ƽ��ʱ��������������干Ϊ5mol������HIΪ1.5mol��������˵����ȷ���ǣ�
��
H2(g)��I2(g)��ƽ��ʱ��������������干Ϊ5mol������HIΪ1.5mol��������˵����ȷ���ǣ�
��
A��ƽ��ʱ����Ϊ2.5mol
B��ƽ��ʱHI�ķֽ���Ϊ20%
C������ʼʱ�������NH4I��������һ������ƽ��ʱ���干Ϊ10 mol
D��������������������������С��V/2 L�����´ﵽƽ��ʱH2��Ũ����ԭƽ���2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ӱ�ʡ������ѧ�ڵ��Ĵ��¿���ѧ�Ծ� ���ͣ�ѡ����
����NH4I����һ�����ΪV L������ܱ������У�������һ���¶Ⱥ����·������з�Ӧ��NH4I��s��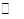 NH3��g����HI��g����2HI��g��
NH3��g����HI��g����2HI��g�� 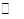 H2��g����I2��g����ƽ��ʱ��������������干Ϊ5 mol������HIΪ1��5 mol��������˵����ȷ���� ��������
H2��g����I2��g����ƽ��ʱ��������������干Ϊ5 mol������HIΪ1��5 mol��������˵����ȷ���� ��������
A��ƽ��ʱ����Ϊ2��5 mol
B��ƽ��ʱHI�ķֽ���Ϊ20%
C������ʼʱ�������NH4I��������һ������ƽ��ʱ���干Ϊ10 mol
D�������������䣬�������������С��L�����´�ƽ��ʱH2��Ũ����ԭƽ���2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����NH4I����һ�����ΪV L������ܱ������У�������һ���¶Ⱥ����·������з�Ӧ��NH4I��s��![]() NH3��g����HI��g����2HI��g��
NH3��g����HI��g����2HI��g�� ![]() H2��g����I2��g����ƽ��ʱ��������������干Ϊ5 mol������HIΪ1��5 mol��������˵����ȷ���� ��������
H2��g����I2��g����ƽ��ʱ��������������干Ϊ5 mol������HIΪ1��5 mol��������˵����ȷ���� ��������
A��ƽ��ʱ����Ϊ2��5 mol
B��ƽ��ʱHI�ķֽ���Ϊ20%
C������ʼʱ�������NH4I��������һ������ƽ��ʱ���干Ϊ10 mol
D�������������䣬�������������С��L�����´�ƽ��ʱH2��Ũ����ԭƽ���2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����NH4I����һ�����ΪV L������ܱ������У�������һ���¶Ⱥ����·������з�Ӧ��NH4I��s��NH3��g����HI��g����2HI��g��
H2��g����I2��g����ƽ��ʱ��������������干Ϊ5 mol������HIΪ1��5 mol��������˵����ȷ���� ��������
A��ƽ��ʱ����Ϊ2��5 mol
B��ƽ��ʱHI�ķֽ���Ϊ20%
C������ʼʱ�������NH4I��������һ������ƽ��ʱ���干Ϊ10 mol
D�������������䣬�������������С��L�����´�ƽ��ʱH2��Ũ����ԭƽ���2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�߰���ѧ�߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ���ѡ��
����NH4I����һ�����ΪVL������ܱ������У�������һ���¶Ⱥ����·������з�Ӧ��NH4I(s) NH3(g)��HI(g)��2HI(g)
NH3(g)��HI(g)��2HI(g) H2(g)��I2(g)��ƽ��ʱ��������������干Ϊ5mol������HIΪ1.5mol��������˵����ȷ���ǣ� ��
H2(g)��I2(g)��ƽ��ʱ��������������干Ϊ5mol������HIΪ1.5mol��������˵����ȷ���ǣ� ��
| A��ƽ��ʱ����Ϊ2.5mol |
| B��ƽ��ʱHI�ķֽ���Ϊ20% |
| C������ʼʱ�������NH4I��������һ������ƽ��ʱ���干Ϊ10 mol |
| D��������������������������С��V/2 L�����´ﵽƽ��ʱH2��Ũ����ԭƽ���2�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com