
ŅŃÖŖĻĀĮŠČČ»Æѧ·½³ĢŹ½£ŗ
¢ŁH2(g)£«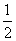 O2(g)===H2O(l) ¦¤H£½£285.8 kJ”¤mol£1
O2(g)===H2O(l) ¦¤H£½£285.8 kJ”¤mol£1
¢ŚH2(g)£«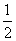 O2(g)===H2O(g) ¦¤H£½£241.8 kJ”¤mol£1
O2(g)===H2O(g) ¦¤H£½£241.8 kJ”¤mol£1
¢ŪC(s)£«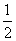 O2(g)===CO(g) ¦¤H£½£110.5 kJ”¤mol£1
O2(g)===CO(g) ¦¤H£½£110.5 kJ”¤mol£1
¢ÜC(s)£«O2(g)===CO2(g) ¦¤H£½£393.5 kJ”¤mol£1
»Ų“šĻĀĮŠø÷ĪŹĢā£ŗ
(1)ÉĻŹö·“Ó¦ÖŠŹōÓŚ·ÅČČ·“Ó¦µÄŹĒ
________________________________________________________________________ӣ
(2)H2µÄČ¼ÉÕČČĪŖ________£»CµÄČ¼ÉÕČČĪŖ________”£
(3)Č¼ÉÕ10 g H2Éś³ÉŅŗĢ¬Ė®£¬·Å³öµÄČČĮæĪŖ________”£
(4)COµÄČ¼ÉÕČČĪŖ________£»ĘäČČ»Æѧ·½³ĢŹ½ĪŖ______________”£
“š°ø””(1)¢Ł¢Ś¢Ū¢Ü””(2)£285.8 kJ”¤mol£1””£393.5 kJ”¤mol£1””(3)1 429.0 kJ””(4)£283.0 kJ”¤mol£1 CO(g)£«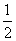 O2(g)===CO2(g)””¦¤H£½£283.0 kJ”¤mol£1
O2(g)===CO2(g)””¦¤H£½£283.0 kJ”¤mol£1
½āĪö””(1)ĖłÓŠČ¼ÉÕ¾łĪŖ·ÅČČ·“Ó¦£¬¢Ł¢Ś¢Ū¢Ü¾łĪŖ·ÅČČ·“Ó¦”£
(2)Č¼ÉÕČČĪŖ1 mol“ææÉČ¼ĪļÉś³ÉĪȶØŃõ»ÆĪļŹ±·Å³öµÄČČĮ棬H2µÄČ¼ÉÕČČĪŖ£285.8 kJ”¤mol£1£¬CµÄČ¼ÉÕČČĪŖ£393.5 kJ”¤mol£1”£
(3)Q·Å£½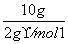 ”Į285.8 kJ”¤mol£1£½1 429.0 kJ”£
”Į285.8 kJ”¤mol£1£½1 429.0 kJ”£
(4)Óɢܣ¢ŪæÉµĆ£¬CO(g)£«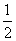 O2(g)===CO2(g)
O2(g)===CO2(g)
¦¤H£½£393.5 kJ”¤mol£1£«110.5 kJ”¤mol£1£½£283.0 kJ”¤mol£1£¬ĘäČ¼ÉÕČČĪŖ£283.0 kJ”¤mol£1”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĄė×Ó·½³ĢŹ½ÓėĖłŹöŹĀŹµĻą·ūĒŅÕżČ·µÄŹĒ £Ø £©
A£®ĻņĆ÷·Æ[KAl(SO4)2]ČÜŅŗÖŠÖšµĪ¼ÓČėBa(OH)2ČÜŅŗÖĮSO42£Ē”ŗĆ³ĮµķĶźČ«£ŗ
2Al3£«£«3SO42££«3Ba2£«£«6OH££½2Al(OH)3”ż£«3BaSO4”ż
B£®ŌŚĒæ¼īČÜŅŗÖŠ“ĪĀČĖįÄĘÓėFe(OH)3·“Ӧɜ³ÉNa2FeO4£ŗ
3ClO”Ŗ+Fe(OH)3= FeO42”Ŗ+3Cl”Ŗ+H2O+H+
C£®ĻņNaAlO2ČÜŅŗÖŠĶØČė¹żĮæCO2ÖĘAl(OH)3£ŗ AlO2-£«CO2£«2H2O= Al(OH)3”ż+HCO3-
D£®Ļņŗ¬ÓŠa mol FeBr2µÄČÜŅŗÖŠ£¬ĶØČėx mol Cl2”£Čōx=a£¬Ōņ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ
2Fe2£«+ 2Br£+ 2Cl2£½Br2 + 2Fe3£«+ 4Cl£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ»ÆŗĻĪļÖŠ¼ČŅ×·¢ÉśČ”“ś·“Ó¦£¬Ņ²æÉ·¢Éś¼Ó³É·“Ó¦£¬»¹ÄÜŹ¹KMnO4ĖįŠŌČÜŅŗĶŹÉ«µÄŹĒ(””””)
A£®ŅŅĶé B£®ŅŅ“¼
C£®¼×±½ D£®±½
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚĻĀĮŠø÷Ėµ·ØÖŠ£¬ÕżČ·µÄŹĒ(””””)
A£®¦¤H>0±ķŹ¾·ÅČČ·“Ó¦£¬¦¤H<0±ķŹ¾ĪüČČ·“Ó¦
B£®ČČ»Æѧ·½³ĢŹ½ÖŠµÄ»Æѧ¼ĘĮæŹżÖ»±ķŹ¾ĪļÖŹµÄĮ棬æÉŅŌŹĒ·ÖŹż
C£®1 mol H2SO4Óė1 mol Ba(OH)2·“Ӧɜ³ÉBaSO4³ĮµķŹ±·Å³öµÄČČ½Š×öÖŠŗĶČČ
D£®1 mol H2Óė0.5 mol O2·“Ó¦·Å³öµÄČČ¾ĶŹĒH2µÄČ¼ÉÕČČ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ£ŗ
¢Ł2C(s)£«O2(g)===2CO(g)
¦¤H£½£221.0 kJ”¤mol£1£»
¢Ś2H2(g)£«O2(g)===2H2O(g)
¦¤H£½£483.6 kJ”¤mol£1”£
ŌņÖʱøĖ®ĆŗĘųµÄ·“Ó¦C(s)£«H2O(g)===CO(g)£«H2(g)µÄ¦¤HĪŖ(””””)
A£®£«262.6 kJ”¤mol£1 B£®£131.3 kJ”¤mol£1
C£®£352.3 kJ”¤mol£1 D£®£«131.3 kJ”¤mol£1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĪŚĀåĶŠĘ·ŌŚŗĻ³É”¢Ņ½Ņ©”¢Č¾ĮĻµČ¹¤ŅµÖŠÓŠ¹ć·ŗÓĆĶ¾£¬Ęä½į¹¹Ź½ČēĶ¼ĖłŹ¾”£½«¼×Č©Ė®ČÜŅŗÓė°±Ė®»ģŗĻÕō·¢æÉÖʵĆĪŚĀåĶŠĘ·”£ČōŌĮĻĶźČ«·“Ӧɜ³ÉĪŚĀåĶŠĘ·£¬Ōņ¼×Č©Óė°±µÄĪļÖŹµÄĮæÖ®±ČĪŖ£Ø £©
A£®1”Ć1 B£®2”Ć3 C£®3”Ć2 D£®2”Ć1
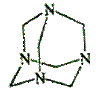
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĄė×ÓÖŠ°ė¾¶×ī“óµÄŹĒ£Ø £©
A£®Na£« B£®Mg2£« C£®O2£ D£® F£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ·Ö×ÓŹ½Ėł±ķŹ¾µÄÓŠ»śĪļÖŠ£¬ĘäŗĖ“Ź²ÕńĒāĘ×æÉÄÜÖ»ĻŌŹ¾Ņ»×éĪüŹÕ·åµÄŹĒ£Ø £©
A.C2H6O B. C4H7Cl C. C3H8 D.C7H8
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠø÷×éÖŠĮ½øö»Æѧ·“Ó¦£¬ŹōÓŚĶ¬Ņ»·“Ó¦ĄąŠĶµÄŅ»×éŹĒ
A£®Óɱ½ÖĘäå±½£»ÓÉŅŅ“¼ÓėŅŅĖį·“Ó¦ÖĘŅŅĖįŅŅõ„
B£®ÓÉŅŅĻ©ÖĘ1,2-¶žäåŅŅĶ飻ÓÉŅŅĶéÖĘŅ»ĀČŅŅĶé
C£®ŅŅĻ©Ź¹äåĖ®ĶŹÉ«£»ŅŅĻ©Ź¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«
D£®Óɱ½ÖĘĻõ»ł±½£»Óɱ½ÖĘ»·¼ŗĶé
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com