
0��ʱ����0.1 mol N2O4����1L�ܱյ���ƿ�У�Ȼ����ƿ����100�� �ĺ��²��У���ƿ�ڵ�������Ϊ����ɫ��N2O4(g) ![]() 2NO2 (g)�����н��۲���˵��������Ӧ�ڸ��������Ѿ��ﵽ��Ӧ�ȵ��ǣ� ��
2NO2 (g)�����н��۲���˵��������Ӧ�ڸ��������Ѿ��ﵽ��Ӧ�ȵ��ǣ� ��
��N2O4������������NO2����������֮��Ϊ1:2��NO2������������NO2����������Ȣ���ƿ�������ѹǿ���ٱ仯����ƿ��������������ٱ仯��NO2�����ʵ���Ũ�Ȳ��ٸı����ƿ���������ɫ���ټ������ƿ�������ƽ����Է����������ٱ仯����ƿ��������ܶȲ��ٱ仯��
A���ڢۢޢ� B���٢ܢ� C��ֻ�Т٢� D��ֻ�Тߢ�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
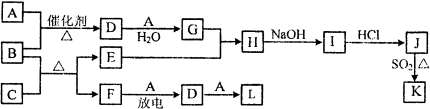
| ʱ��/s | 0 | 30 | 60 | 90 |
| n��L��/mol | 0.80 | a | b | c |
| n��M��/mol | 0.00 | 0.10 | 0.20 | 0.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.1 mol��þ�������������100mL 2mol/L H2SO4��Һ�У�Ȼ���ٵμ�1 mol/L NaOH��Һ����������ش�
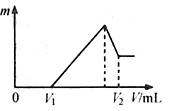
��1��д�������йص����ӷ���ʽ��
��2�����ڵμ�NaOH��Һ�����У���������m�����NaOH��Һ�����V�仯��ͼ��ʾ����V1��160mLʱ���������ĩ��n(Mg)�� mol��
V2�� mL����������������� g��
��3�����ڵμ�NaOH��Һ�����У���ʹMg2����Al3���պó�����ȫ�������NaOH��Һ�����V(NaOH)= mL��
��4�����������Ϊ0.1 mol������Mg�۵����ʵ�������Ϊa����100 mL 2 mol/L�������ܽ�˻������ټ���450 mL 1mol/L��NaOH��Һ�����ó�������Al(OH)3�������������a��ȡֵ��Χ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.1 mol��þ�������������100mL 2mol/L H2SO4��Һ�У�Ȼ���ٵμ�1 mol/L NaOH��Һ����������ش�
��1��д�������йص����ӷ���ʽ��
��2�����ڵμ�NaOH��Һ�����У���������m�����NaOH��Һ�����V�仯��ͼ��ʾ����V1��160mLʱ���������ĩ��n(Mg)�� mol��
V2�� mL����������������� g��
��3�����ڵμ�NaOH��Һ�����У���ʹMg2����Al3���պó�����ȫ�������NaOH��Һ�����V(NaOH)= mL��
��4�����������Ϊ0.1mol������Mg�۵����ʵ�������Ϊa����100 mL 2mol/L�������ܽ�˻������ټ���450 mL 1mol/L��NaOH��Һ�����ó�������Al(OH)3�������������a��ȡֵ��Χ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��㶫ʡտ��һ�и�����ѧ��10�·��¿������ۣ���ѧ�� ���ͣ������
��0.1 mol��þ�������������100mL 2mol/L H2SO4��Һ�У�Ȼ���ٵμ�1 mol/L NaOH��Һ����������ش�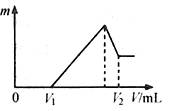
��1��д�������йص����ӷ���ʽ��
��2�����ڵμ�NaOH��Һ�����У���������m�����NaOH��Һ�����V�仯��ͼ��ʾ����V1��160mLʱ���������ĩ��n(Mg)�� mol��
V2�� mL����������������� g��
��3�����ڵμ�NaOH��Һ�����У���ʹMg2����Al3���պó�����ȫ�������NaOH��Һ�����V(NaOH)= mL��
��4�����������Ϊ0.1 mol������Mg�۵����ʵ�������Ϊa����100 mL 2 mol/L�������ܽ�˻������ټ���450 mL 1mol/L��NaOH��Һ�����ó�������Al(OH)3�������������a��ȡֵ��Χ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��㶫ʡ������ѧ��10�·��¿������ۣ���ѧ�� ���ͣ������
��0.1 mol��þ�������������100mL 2mol/L H2SO4��Һ�У�Ȼ���ٵμ�1 mol/L NaOH��Һ����������ش�

��1��д�������йص����ӷ���ʽ��
��2�����ڵμ�NaOH��Һ�����У���������m�����NaOH��Һ�����V�仯��ͼ��ʾ����V1��160mLʱ���������ĩ��n(Mg)�� mol��
V2�� mL����������������� g��
��3�����ڵμ�NaOH��Һ�����У���ʹMg2����Al3���պó�����ȫ�������NaOH��Һ�����V(NaOH)= mL��
��4�����������Ϊ0.1 mol������Mg�۵����ʵ�������Ϊa����100 mL 2 mol/L�������ܽ�˻������ټ���450 mL 1mol/L��NaOH��Һ�����ó�������Al(OH)3�������������a��ȡֵ��Χ�ǣ� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com