
£Ø1£©ŹµŃéŹŅÓĆĀČ»Æļ§¹ĢĢåÖĘČ”°±ĘųµÄ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø2£©½«4.48L£Ø±ź×¼×“æö£©°±ĘųĶØČėĖ®ÖŠµĆµ½0.05LČÜŅŗ£¬ĖłµĆČÜŅŗµÄĪļÖŹµÄĮæÅØ¶ČŹĒ ”£
£Ø3£©ĻÖÓŠ100mL AlCl3ÓėMgSO4µÄ»ģŗĻČÜŅŗ£¬·Ö³ÉĮ½µČ·Ż”£
¢ŁĻņĘäÖŠŅ»·ŻÖŠ¼ÓČė10mL 4mol/LµÄ°±Ė®£¬Ē”ŗĆĶźČ«·“Ó¦£¬ĘäÖŠAlCl3Óė°±Ė®·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ”£¼ĢŠų¼ÓČėl mol/L NaOHČÜŅŗÖĮ10mLŹ±£¬³Įµķ²»ŌŁ¼õÉŁ£¬³Įµķ¼õÉŁµÄĄė×Ó·½³ĢŹ½ŹĒ £¬¼õÉŁµÄ³ĮµķµÄĪļÖŹµÄĮæŹĒ ”£
¢ŚĻņĮķŅ»·ŻÖŠ¼ÓČėa mL 1mol/LBaCl2ČÜŅŗÄÜŹ¹SO42£³ĮµķĶźČ«£¬a= ”£
£Ø1£©Ca£ØOH£©2£«2NH4Cl CaCl2£«2H2O£«2NH3”ü
CaCl2£«2H2O£«2NH3”ü
£Ø2£©4 mol/L £Ø1·Ö£©
£Ø3£©¢ŁA13+£«3NH3”¤H2O=Al£ØOH£©3”ż£«3NH4+
Al£ØOH£©3£«OH£=AlO2££«2H2O
0.01 mol
¢Ś 5
½āĪö ŹŌĢā·ÖĪö£ŗ¢ÅŹµŃéŹŅÓĆĀČ»Æļ§¹ĢĢåÖĘČ”°±ĘųµÄ»Æѧ·½³ĢŹ½ŹĒCa£ØOH£©2£«2NH4Cl
ŹŌĢā·ÖĪö£ŗ¢ÅŹµŃéŹŅÓĆĀČ»Æļ§¹ĢĢåÖĘČ”°±ĘųµÄ»Æѧ·½³ĢŹ½ŹĒCa£ØOH£©2£«2NH4Cl CaCl2£«2H2O£«2NH3”ü
CaCl2£«2H2O£«2NH3”ü
¢Ęøł¾Żc=n/V,µĆ°±ĘųµÄĪļÖŹµÄĮæŹĒ4.48L/22.4L/mol=0.2mol£¬ĖłŅŌĖłµĆČÜŅŗĪļÖŹµÄĮæÅضČĪŖ£ŗ0.2mol/0.05L="4" mol/L
¢Ē¢ŁAlCl3Óė°±Ė®·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒA13+£«3NH3”¤H2O=Al£ØOH£©3”ż£«3NH4+£¬³Įµķ¼õÉŁ¼“ĒāŃõ»ÆĀĮČÜÓŚĒāŃõ»ÆÄĘČÜŅŗ£¬Ąė×Ó·½³ĢŹ½ĪŖAl£ØOH£©3£«OH£=AlO2££«2H2O£¬³Įµķ¼õÉŁµÄĪļÖŹµÄĮ漓ĪŖĒāŃõ»ÆÄʵÄĪļÖŹµÄĮæ0.01mol
¢ŚÓÉ¢ŁÖŖAl(OH)3µÄĪļÖŹµÄĮæĪŖ0.01mol£¬ĻūŗÄNH3”¤H2O0.03mol£¬ĖłŅŌMg2+ĻūŗÄNH3”¤H2O£Ø0.04mol-0.03mol£©=0.01mol,Ōņn£ØMg2+£©=0.005mol=n(SO42-£©£¬ĖłŅŌŠčŅŖ1mol/LBaCl2ČÜŅŗ5mlŹ¹SO42-³ĮµķĶźČ«”£
æ¼µć£ŗĄė×Ó·“Ó¦¼°Ļą¹Ų¼ĘĖć



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(1)ÓÉČżøöŌ×Ó¹¹³ÉµÄijĘųĢå·Ö×Ó£¬ĘäĦ¶ūÖŹĮæĪŖM g/mol£¬øĆĘųĢåµÄĢå»żĪŖV L(±ź×¼
דæö)£¬Éč°¢·ü¼ÓµĀĀŽ³£ŹżµÄÖµĪŖNA£¬Ōņ£ŗ
¢ŁøĆĘųĢåµÄĪļÖŹµÄĮæĪŖ________mol£»
¢ŚøĆĘųĢåÖŠĖłŗ¬µÄŌ×Ó×ÜŹżĪŖ________øö£»
¢ŪøĆĘųĢåŌŚ±ź×¼×“æöĻĀµÄĆܶČĪŖ________g/L£»
¢ÜøĆĘųĢåµÄŅ»øö·Ö×ÓµÄÖŹĮæĪŖ________g”£
(2)ĘųĢå»ÆŗĻĪļA·Ö×ÓŹ½æɱķŹ¾ĪŖOxFy£¬ŅŃÖŖĶ¬ĪĀĶ¬Ń¹ĻĀ10 mL AŹÜČČ·Ö½āÉś³É15 mL O2ŗĶ10 mL F2£¬ŌņAµÄ»ÆѧŹ½ĪŖ________£¬ĶʶĻµÄŅĄ¾ŻĪŖ______________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijŠ£»ÆѧŠ”×éѧɜ½ųŠŠ”°ĘųĢåĻą¶Ō·Ö×ÓÖŹĮæµÄ²ā¶Ø”±µÄŹµŃ锣²Ł×÷ČēĻĀ£ŗÓĆÖŹĮæŗĶČŻ»ż¶¼ĻąµČµÄÉÕĘæŹÕ¼ÆĘųĢ壬³ĘĮæŹÕ¼ÆĀśĘųĢåµÄÉÕĘæÖŹĮ棬Źż¾Ż¼ūĻĀ±ķ(ŅŃ»»Ėć³É±ź×¼×“æöĻĀµÄŹżÖµ)”£
| ĘųĢå | ÉÕĘæŗĶĘųĢåµÄ×ÜÖŹĮæ(g) |
| A | 48.4082 |
| B | 48.4082 |
| C | 48.4082 |
| D | 48.4342 |
| E | 48.8762 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
±ź×¼×“æöĻĀ336 LµÄNH3ČÜÓŚ1 LĖ®ÖŠ£¬ĖłµĆČÜŅŗµÄÖŹĮæ·ÖŹżĪŖ________£¬ČōøĆČÜŅŗµÄĆܶČĪŖa g/cm3£¬ŌņĪļÖŹµÄĮæÅضČĪŖ________”£½«ÉĻŹö°±Ė®Č«²æ×Ŗ»ÆĪŖNH4Cl£¬ĖłŠč4 mol”¤L£1µÄŃĪĖįµÄĢå»żĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĮņĖįµÄ¹¤ŅµÖʱøŹĒŅ»øöÖŲŅŖµÄ»Æ¹¤Éś²ś¹ż³Ģ£¬µ«ŌŚÉś²ś¹ż³ĢÖŠ»į²śÉś“óĮæĪŪČ¾£¬ŠčŅŖŌŚÉś²ś¹¤ŅÕÖŠæ¼ĀĒµ½ĀĢÉ«¹¤ŅÕ”£
IĪ²ĘųµÄĪüŹÕŗĶ×ŪŗĻĄūÓĆ”£
ŅŌ¹¤ŅµÖĘĮņĖįµÄĪ²Ęų”¢°±Ė®”¢ŹÆ»ŅŹÆ”¢½¹Ģ攢Ģ¼ĖįĀČļ§ŗĶKCIĪŖŌĮĻæÉŅŌŗĻ³ÉĮņ»ÆøĘ”¢ĮņĖį¼Ų”¢ŃĒĮņĖįļ§µČĪļÖŹ”£ŗĻ³ÉĀ·ĻßČēĻĀ£ŗ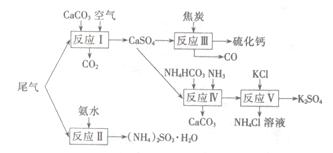
£Ø1£©·“Ó¦IIIÖŠŃõ»Æ¼ĮÓė»¹Ō¼ĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ ”£
£Ø2£©·“Ó¦¢ōµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©·“Ó¦VŌŚ25”ę”¢40%µÄŅŅ¶ž“¼ČÜŅŗÖŠ½ųŠŠ£¬øĆ·“Ó¦ÄÜĖ³Ąū½ųŠŠµÄŌŅņĪŖ ”£
¢ņ“߻ƼĮµÄ»ŲŹÕĄūÓĆ”£
SO2µÄ“ß»ÆŃõ»ÆĖłŹ¹ÓĆµÄ“ß»Æ¼ĮĪŖV2O5£¬Źµ¼ŹÉś²śÖŠ£¬“߻ƼĮŌŚŹ¹ÓĆŅ»¶ĪŹ±¼äŗ󣬻įŗ¬ÓŠV2O5”¢VOSO4ŗĶSiO2µČ£¬ĘäÖŠVOSO4”£ÄÜČÜÓŚĖ®”£»ŲŹÕV2O5£¬µÄÖ÷ŅŖĮ÷³ĢČēĻĀ£ŗ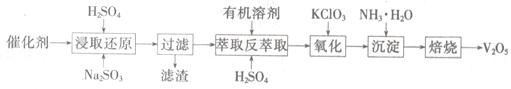
£Ø4£©Čō·“ŻĶČ”Ź¹ÓƵÄĮņĖįÓĆĮæ¹ż“󣬽ųŅ»²½“¦ĄķŹ±»įŌö¼Ó____ µÄÓĆĮ攣
£Ø5£©½žČ”»¹Ō¹ż³ĢµÄ²śĪļÖ®Ņ»ŹĒVOSO4£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
Ńõ»Æ¹ż³ĢµÄ»Æѧ·½³ĢŹ½ĪŖKClO3+6VOSO4+3H2SO4= 2(VO)2(SO4)3+KCl+3H2O£»ČōĮ½²½ĖłÓĆŹŌ¼ĮNa2SO3ÓėKC1O3µÄĪļÖŹµÄĮæÖ®±ČĪŖ12£ŗ7£¬ŌņøĆ“ß»Æ¼ĮÖŠV2O5”¢VOSO4µÄĪļÖŹµÄĮæÖ®±ČĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¹żŃõ»ÆĒāČÜŅŗĖ×ĆūĖ«ŃõĖ®,Ņ½ĮĘÉĻĄūÓĆĖüӊɱ¾śĻū¶¾×÷ÓĆĄ“ĒåĻ“ÉĖæŚ”£»Ų“šĻĀĮŠÓŠ¹ŲĖ«ŃõĖ®µÄĪŹĢā:
(1)ĻĀŹö·“Ó¦ÖŠ,H2O2½öĢåĻÖŃõ»ÆŠŌµÄ·“Ó¦ŹĒ””””””””(Ģī“śŗÅ)”£
A£®Na2O2+2HCl 2NaCl+H2O2 2NaCl+H2O2 |
B£®Ag2O+H2O2 2Ag+O2”ü+H2O 2Ag+O2”ü+H2O |
C£®2H2O2 2H2O+O2”ü 2H2O+O2”ü |
D£®3H2O2+Cr2(SO4)3+10KOH 2K2CrO4+3K2SO4+8H2O 2K2CrO4+3K2SO4+8H2O |

 A+NH3”ü,ŹŌÖø³öÉś³ÉĪļAµÄ»ÆѧŹ½ĪŖ”””””””””””””””””£
A+NH3”ü,ŹŌÖø³öÉś³ÉĪļAµÄ»ÆѧŹ½ĪŖ”””””””””””””””””£ ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijĶ¬Ń§¹ŗĀņĮĖŅ»Ęæ”Į”ĮÅĘ”°84Ļū¶¾Ņŗ”±£¬²éŌÄĻą¹Ų׏ĮĻŗĶĻū¶¾Ņŗ°ü×°ĖµĆ÷µĆµ½ČēĻĀŠÅĻ¢£ŗ
”°84Ļū¶¾Ņŗ”±£ŗŗ¬25%NaClO 1 000 mL”¢ĆܶČ1.19 g”¤cm£3£¬Ļ”ŹĶ100±¶(Ģå»ż±Č)ŗóŹ¹ÓĆ”£
Ēėøł¾ŻŅŌÉĻŠÅĻ¢ŗĶĻą¹ŲÖŖŹ¶»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)øĆ”°84Ļū¶¾Ņŗ”±µÄĪļÖŹµÄĮæÅضČĪŖ________ mol”¤L£1”£
(2)øĆĶ¬Ń§Č”100 mLøĆ”°84Ļū¶¾Ņŗ”±Ļ”ŹĶŗóÓĆÓŚĻū¶¾£¬Ļ”ŹĶŗóµÄČÜŅŗÖŠc(Na£«)£½________ mol”¤L£1(¼ŁÉčĻ”ŹĶŗóČÜŅŗĆܶČĪŖ1.0 g”¤cm£3)”£
(3)ijŹµŃéŠčÓĆ480 mLŗ¬25%NaClOµÄĻū¶¾Ņŗ”£øĆĶ¬Ń§²ĪŌÄøĆ”°84Ļū¶¾Ņŗ”±µÄÅä·½£¬ÓūÓĆNaClO¹ĢĢåÅäÖĘøĆĻū¶¾Ņŗ”£
¢ŁĻĀĮŠĖµ·ØÕżČ·µÄŹĒ________”£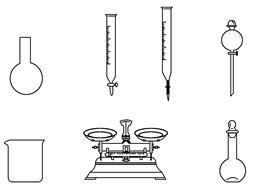
A£®ČēÉĻĶ¼ĖłŹ¾µÄŅĒĘ÷ÖŠ£¬ÓŠĖÄÖÖŹĒ²»ŠčŅŖµÄ£¬»¹ŠčŅ»ÖÖ²£Į§ŅĒĘ÷
B£®ČŻĮæĘæÓĆÕōĮóĖ®Ļ“¾»ŗó£¬Ó¦ŗęøɲÅÄÜÓĆÓŚČÜŅŗÅäÖĘ
C£®ĄūÓĆ¹ŗĀņµÄÉĢĘ·NaClOĄ“ÅäÖĘæÉÄܵ¼ÖĀ½į¹ūĘ«µĶ
D£®ŠčŅŖ³ĘĮæµÄNaClO¹ĢĢåÖŹĮæĪŖ143 g
¢ŚŌŚÅäÖĘ¹ż³ĢÖŠ£¬ĻĀĮŠ²Ł×÷æÉÄÜŹ¹ÅäÖʵÄČÜŅŗµÄÅضČĘ«“óµÄŹĒ________”£
A£®ÉÕ±ÖŠČÜŅŗ×ŖŅʵ½ČŻĮæĘæÖŠŹ±£¬Ī“Ļ“µÓÉÕ±
B£®¶ØČŻŹ±£¬ø©ŹÓæĢ¶ČĻß
C£®¶ØČŻŹ±£¬ŃöŹÓæĢ¶ČĻß
D£®ŅĘŅŗŹ±£¬ÓŠÉŁĮæŅŗĢ彦³ö
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŹµŃéŹŅÅäÖĘ480 mL 0£®1mol·L-1 NaOHČÜŅŗ£¬»Ų“šĻĀĮŠĪŹĢā
£Ø1£©Ó¦ÓĆĶŠÅĢĢģĘ½³ĘČ”ĒāŃõ»ÆÄĘ¹ĢĢå g”£
£Ø2£©ÅäÖĘNaOHČÜŅŗŹ±ŠčÓƵÄÖ÷ŅŖŅĒĘ÷ÓŠĶŠÅĢĢģĘ½”¢Ņ©³×”¢ÉÕ±”¢²£Į§°ō”¢ĮæĶ² ”¢ ”£
Ź¹ÓĆČŻĮæĘæĒ°±ŲŠė½ųŠŠµÄ²Ł×÷ŹĒ ”£
£Ø3£©ÅäÖĘŹ±£¬øĆŹµŃéĮ½“ĪÓƵ½²£Į§°ō£¬Ęä×÷ÓĆ·Ö±šŹĒ ”¢ ”£
£Ø4£©¶ØČŻŹ±Čō¼ÓĖ®³¬¹żæĢ¶ČĻߣ¬Ó¦ČēŗĪ“¦Ąķ£æ ”£
£Ø5£©ŹŌ·ÖĪöĻĀĮŠ²Ł×÷¶ŌĖłÅäČÜŅŗµÄÅضČÓŠŗĪÓ°Ļģ”£
A£® Ę«øß B£® Ę«µĶ C£®²»±ä£ØÓĆ·ūŗÅ»Ų“š£©
¢Ł ¶ØČŻŹ±ø©ŹÓæĢ¶ČĻß £»
¢Ś ·ÅŌŚĀĖÖ½ÉĻ³ĘĮæNaOH¹ĢĢå ”£
¢ŪČŻĮæĘæƻӊøÉŌļ£¬ÓŠÉŁĮæÕōĮóĖ®£ŗ ”£
¢Ü¶ØČŻŗ󣬼ÓøĒµ¹×ŖŅ”ŌČŗ󣬷¢ĻÖŅŗĆęµĶÓŚæĢ¶ČĻߣ¬ÓÖµĪ¼ÓÕōĮóĖ®ÖĮæĢ¶Č£ŗ ”£
£Ø6£©ĒėÄć°ļÖś°ŃŹŌ¼ĮĘæ£ØŹ¢·ÅÉĻŹöÅäÖĘŗƵÄČÜŅŗ£©ÉĻ±źĒ©µÄÄŚČŻĢīÉĻČ„ (±źĒ©ČēĶ¼)”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com