
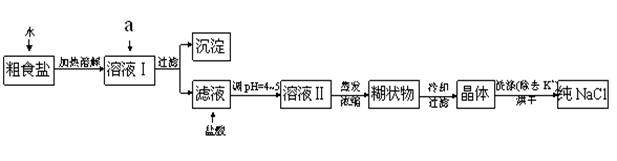
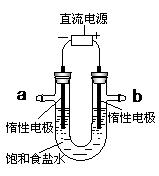


 Č«ÓÅ漵䵄ŌŖ¼ģ²ā¾ķ¼°¹éĄą×Üø“Ļ°ĻµĮŠ“š°ø
Č«ÓÅ漵䵄ŌŖ¼ģ²ā¾ķ¼°¹éĄą×Üø“Ļ°ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| A£®¢Ū¢Ś¢Ł¢Ż¢Ü | B£®¢Ł¢Ś¢Ū¢Ż¢Ü | C£®¢Ś¢Ū¢Ł¢Ż¢Ü | D£®¢Ū¢Ż¢Ś¢Ł¢Ü |
 £Ø2£©ČēŗĪÓĆŹµŃé·½·ØČ·¶Ø¢ŁÖŠNa2CO3ČÜŅŗŅŃ¹żĮæ£æ
£Ø2£©ČēŗĪÓĆŹµŃé·½·ØČ·¶Ø¢ŁÖŠNa2CO3ČÜŅŗŅŃ¹żĮæ£æ ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¼ÓČė±½Ź¹Ö®·“Ó¦ | B£®¼ÓČė½Ļ“óĮæNaOHČÜŅŗŗó¾²ÖĆ·ÖŅŗ |
| C£®¼ÓČėKIČÜŅŗ | D£®¼ÓČė½Ļ“óĮæµÄCCl4ŻĶČ”¾²ÖĆŗó·ÖŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
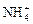 ”¢H£«”¢Cl£”¢
”¢H£«”¢Cl£”¢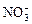 ”¢
Ӣ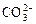 Ӣ
Ӣ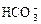 Ӣ
”¢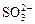 ”¢OH£”£ÉĻŹöĄė×ÓÖŠ£¬ŌŚĒæĖįŠŌČÜŅŗĄļ²»æÉÄÜ“óĮæ“ęŌŚµÄŹĒ____________£»ŌŚĒæ¼īŠŌČÜŅŗÖŠ²»æÉÄÜ“óĮæ“ęŌŚµÄĄė×ÓÓŠ____________£»¼Č²»ÄÜŌŚĒæĖįŠŌČÜŅŗĄļ“óĮæ“ęŌŚ£¬Ņ²²»ÄÜŌŚĒæ¼īŠŌČÜŅŗĄļ“óĮæ“ęŌŚµÄŹĒ______£»ĪŽĀŪŌŚĒæĖįŠŌČÜŅŗ»¹ŹĒĒæ¼īŠŌČÜŅŗĄļ£¬¶¼æÉŅŌ“óĮæ“ęŌŚµÄŹĒ____________
”¢OH£”£ÉĻŹöĄė×ÓÖŠ£¬ŌŚĒæĖįŠŌČÜŅŗĄļ²»æÉÄÜ“óĮæ“ęŌŚµÄŹĒ____________£»ŌŚĒæ¼īŠŌČÜŅŗÖŠ²»æÉÄÜ“óĮæ“ęŌŚµÄĄė×ÓÓŠ____________£»¼Č²»ÄÜŌŚĒæĖįŠŌČÜŅŗĄļ“óĮæ“ęŌŚ£¬Ņ²²»ÄÜŌŚĒæ¼īŠŌČÜŅŗĄļ“óĮæ“ęŌŚµÄŹĒ______£»ĪŽĀŪŌŚĒæĖįŠŌČÜŅŗ»¹ŹĒĒæ¼īŠŌČÜŅŗĄļ£¬¶¼æÉŅŌ“óĮæ“ęŌŚµÄŹĒ____________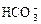 £«______£½
£«______£½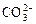 £«______£»
£«______£»²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 ½ųŠŠĮĖŹµŃéĻÖĻóµÄ¼ŁÉč£¬ĒėÄć°ļÖśĖū¶ŌÕāŠ©¼ŁÉč×÷Ņ»Š©ÅŠ¶Ļ£ŗ
½ųŠŠĮĖŹµŃéĻÖĻóµÄ¼ŁÉč£¬ĒėÄć°ļÖśĖū¶ŌÕāŠ©¼ŁÉč×÷Ņ»Š©ÅŠ¶Ļ£ŗ| ŹµŃé²½Öč | ŹµŃéĻÖĻó | ÅŠ ¶Ļ |
| Č”¹ĢĢåČÜÓŚĖ® | ¢ŁČōÖ»µĆµ½Ą¶É«ČÜŅŗ | Ņ»¶Øƻӊ____________________________”£ |
| ¢ŚČōÓŠ°×É«³Įµķ£¬¾²ÖĆ£¬ÉĻ²ćČÜŅŗĪŽÉ« | Ņ»¶Øƻӊ___________________________”£ | |
| Č”¢ŚµÄ»ģ ŗĻĪļ¹żĀĖ | ¢ŪŌŚ³ĮµķÖŠ¼ÓČėŃĪĖį£¬³Įµķ²æ·ÖČܽā£»¹żĀĖ£¬ŌŚĀĖŅŗÖŠ¼ÓČėAgNO3ČÜŅŗŗĶĻ”ĻõĖį£¬ÓŠ°×É«³Įµķ | Ņ»¶Øŗ¬ÓŠ_____________________________£¬ ³ĮµķÖŠ¼ÓČėŃĪĖįµÄĄė×Ó·½³ĢŹ½£ŗ _______________________________________”£ ÄÜ·ńČ·¶ØŌ¹ĢĢåÖŠŹĒ·ńŗ¬ÓŠNaCl£¬²¢ĖµĆ÷ĄķÓÉ _______________________________________ _______________________________________”£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¹żĀĖ | B£®ÕōĮó | C£®Õō·¢ | D£®ŻĶČ” |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| | ĪļÖŹ | ŌÓÖŹ | ŹŌ¼Į | Ö÷ŅŖ²Ł×÷ |
| A | CH3CH2OH | H2O | Na | ÕōĮó |
| B | SiO2 | Fe2O3 | ŃĪĖį | ¹żĀĖ |
| C | I2 | H2O | ŅŅ“¼ | ŻĶČ” |
| D | H2O | Fe3£« | NaOH | ¹żĀĖ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¹żĀĖ | B£®ÕōĮó | C£®·ÖŅŗ | D£®ŻĶČ” |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

 ĪöĢÖĀŪ£ŗ
ĪöĢÖĀŪ£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com