
��ͼ��ʾ�����Թܷ���ʢ��25��ʱ����ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸С��þƬ�����õιܵ���5 mL�������Թ��У�
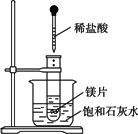
������������⣺
(��ʾ���������Ƶ��ܽ�����¶����߶�����)
(1)ʵ���й۲쵽��������_______________��
(2)�������������ԭ����_______________��
(3)д���йط�Ӧ�����ӷ���ʽ��_________��
(4)��ʵ����֪��MgCl2��Һ��H2��������________(����ڡ���С�ڡ����ڡ�)þƬ���������������
|
�����𰸣�(1)�Թ��������ݲ���������ʯ��ˮ���а�ɫ�������� ����(2)þƬ����ϡ����ų��������Ҵ˷�ӦΪ���ȷ�Ӧ�����¶����ߣ�Ca(OH)2���ܽ�ȱ�С ����(3)Mg��2H+ ����(4)С�� ����˼·������þ������ķ�Ӧ�������������ȣ������Թ��������ݲ������ձ��еı���ʯ��ˮ�����¶����߶������������ƣ��ɴ˿�֪þ��������������������ɵ��������Ȼ�þ���������� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ʾ�����Թܷ���ʢ25��ʱ����ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸С��þƬ�����õιܵ���5mL�������Թ��У�
��ͼ��ʾ�����Թܷ���ʢ25��ʱ����ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸С��þƬ�����õιܵ���5mL�������Թ��У��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ʾ�����Թܷ���ʢ��25����履��ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸��þ�������õιܵ���5mL���������Թ��У��Իش��������⣺����ʾ��Ca��OH��2�ܽ�������¶ȵ����߶���С��
��ͼ��ʾ�����Թܷ���ʢ��25����履��ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸��þ�������õιܵ���5mL���������Թ��У��Իش��������⣺����ʾ��Ca��OH��2�ܽ�������¶ȵ����߶���С���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
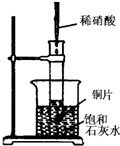 ��ͼ��ʾ�����Թܷ���ʢ��25��ʱ����ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸С��ͭƬ�����õιܵ���10mLϡ���ᣮ�ݴ˻ش��������⣺
��ͼ��ʾ�����Թܷ���ʢ��25��ʱ����ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸С��ͭƬ�����õιܵ���10mLϡ���ᣮ�ݴ˻ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)ʵ���й۲쵽��������_______________________________________________��
(2)�������������ԭ����_______________________________________________��
(3)д���йط�Ӧ�����ӷ���ʽ____________________________________________��
(4)��ʵ����֪��MgCl2��Һ��H2��������_____________(����ڡ���С�ڡ������ڡ�)þƬ���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ�����Թܷ���ʢ��25��ʱ����ʯ��ˮ���ձ��У�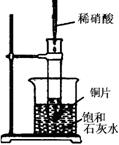
�Թ��п�ʼ���뼸С��ͭƬ�����õιܵ���10mLϡ���ᡣ�ݴ˻ش��������⣺
(1)ʵ���й۲쵽�������� ��
(2)�������������ԭ���� ��
(3)д���йط�Ӧ�Ļ�ѧ����ʽ ��
(4)��ʵ����֪����Ӧ������������ (����ڡ�����С�ڡ��������ڡ�)�����ͭƬ����������
��5����װ�������ԵIJ��㣬ԭ���� ���Ľ��ķ����� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com