
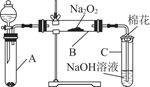
| ŹµŃé²½Öč | Ō¤ĘŚĻÖĻóŗĶ½įĀŪ |
| ²½Öč1£ŗČ”BÖŠµÄÉŁĮæ¹ĢĢåѳʷӌŹŌ¹ÜÖŠ£¬µĪ¼Ó×ćĮæÕōĮóĖ®£¬Čܽā£¬Č»ŗóȔɣĮæ“ż²āŅŗ·Ö±šÖĆÓŚ¢ń”¢¢ņŹŌ¹ÜÖŠ | ¹ĢĢåĶźČ«Čܽā |
| ²½Öč2£ŗĶł¢ńŹŌ¹ÜÖŠ¼ÓČė £¬ŌŁµĪ¼Ó | £¬ |
| ŌņÖ¤Ć÷Éś³ÉĪļÖŠŗ¬Na2SO4 | |
| ²½Öč3£ŗĶł¢ņŹŌ¹ÜÖŠ | |
| | Čō £¬ |
| ŌņÖ¤Ć÷Éś³ÉĪļÖŠÓŠNa2SO3£»Čō | |
| | |
| ŌņĖµĆ÷Éś³ÉĪļ֊ƻӊNa2SO3”£ | |
| ²½Öč2£ŗĶł¢ńŹŌ¹ÜÖŠ¼ÓČė×ćĮæµÄ1_mol”¤L£1ŃĪĖį£¬ŌŁµĪ¼Ó1_mol”¤L£1_BaCl2ČÜŅŗ | ÓŠ°×É«³ĮµķÉś³É£¬ŌņÖ¤Ć÷Éś³ÉĪļÖŠŗ¬Na2SO4 |
| ²½Öč3£ŗĶł¢ņŹŌ¹ÜÖŠ¼ÓČė2”«3µĪ0.01_mol”¤L£1_KMnO4ĖįŠŌČÜŅŗ£¬Õńµ“ | ČōKMnO4ČÜŅŗ×ĻŗģÉ«ĶŹČ„£¬ŌņÖ¤Ć÷Éś³ÉĪļÖŠÓŠNa2SO3£» |
| ČōKMnO4ČÜŅŗ×ĻŗģÉ«²»ĶŹČ„£¬ŌņĖµĆ÷Éś³ÉĪļ֊ƻӊNa2SO3 | |
 ”Į100%
”Į100% ”Į100%£½
”Į100%£½ ”Į100%”£
”Į100%”£

 ÓżӾ«¾ķĻµĮŠ“š°ø
ÓżӾ«¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ČżøöÉÕ±ÖŠŅ»¶Ø¾ł»į·¢ÉśµÄĄė×Ó·“Ó¦ÓŠ£ŗ2Na£«2H2O=2Na£«£«2OH££«H2”ü |
| B£®ČżøöÉÕ±ÖŠÄĘ¾łŌŚŅŗĆęÉĻ¾ēĮŅ·“Ó¦£¬Ļą±Č¶ųŃŌ£¬XÉÕ±ÖŠµÄ·“Ó¦Ę½»ŗŠ© |
| C£®ZÉÕ±ÖŠŅ»¶Ø»įÓŠ³ĮµķÉś³É£¬µ«³Įµķ²»ŹĒµ„ÖŹĶ |
| D£®ČżøöÉÕ±ÖŠÖĆ»»Éś³ÉĘųĢåµÄĪļÖŹµÄĮæŅ»¶ØĻąĶ¬ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
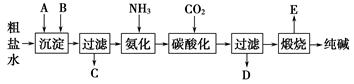
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¼ī½šŹōŌ×Ó×īĶā²ć¶¼Ö»ÓŠ1øöµē×Ó£¬ŌŚ»Æѧ·“Ó¦ÖŠČŻŅ׏§Č„×īĶā²ćÕāøöµē×Ó |
| B£®¶¼ŹĒĒ滹Ō¼Į |
| C£®¶¼ÄÜŌŚO2ÖŠČ¼ÉÕÉś³É¹żŃõ»ÆĪļ |
| D£®¶¼ÄÜÓėĖ®·“Ӧɜ³ÉĒæ¼ī |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ö»ÓŠ¢Ł¢Ś | B£®Ö»ÓŠ¢Ū | C£®Ö»ÓŠ¢Ś¢Ū | D£®¢Ł¢Ś¢Ū |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¼ÓČČ£¬æ“ÓŠĪŽĘųĢå·Å³ö |
| B£®µĪ¼ÓŃĪĖį£¬æ“ÓŠĪŽĘųĢå·Å³ö |
| C£®ČÜÓŚĖ®ŗ󣬵Ī¼ÓĻ”µÄĀČ»Æ±µČÜŅŗæ“ÓŠĪŽ°×É«³ĮµķÉś³É |
| D£®ČÜÓŚĖ®ŗ󣬵Ī¼Ó³ĪĒåŹÆ»ŅĖ®æ“ÓŠĪŽ°×É«³ĮµķÉś³É |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ö»ÓŠ¢Ł¢Ü | B£®Ö»ÓŠ¢Ū | C£®Ö»ÓŠ¢Ś¢Ū | D£®Ö»ÓŠ¢Ł¢Ū¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®9.2g | B£®10.6g | C£®6.2g | D£®4.6g |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com