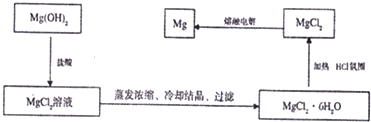
ΒΣ―θΜ·ΚœΈο «¥σΤχΈέ»ΨΒΡ÷Ί“Σ“ρΥΊΘ§ ΙNO
xΒΡ”––ßœϊ≥ΐ≥…ΈΣΜΖ±ΘΝλ”ρΒΡ÷Ί“ΣΩΈΧβΘ°

Θ®1Θ©Τϊ≥Β≈≈Ζ≈ΒΡΈ≤Τχ÷–Κ§NOΘ§…ζ≥…NOΒΡΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ
Θ°
Θ®2Θ©ΫΪNO
2±δ≥…ΈόΚΠΒΡN
2“Σ’“ΒΫ ΚœΒΡΈο÷ G”κ Β±ΒΡΖ¥”ΠΧθΦΰΘ§G”ΠΈΣ
Θ®Χν–¥ΓΑ―θΜ·ΦΝΓ±ΜρΓΑΜΙ‘≠ΦΝΓ±Θ©Θ°œ¬ Ϋ÷–X±Ί–κΈΣΈόΈέ»ΨΒΡΈο÷ Θ§œΒ ΐnΩ…“‘ΈΣ0Θ°
NO
2+G
N
2+H
2O+nXΘ®Έ¥≈δΤΫΒΡΖ¥”Π ΫΘ©Θ°
œ¬Ν–Μ·ΚœΈο÷–Θ§¬ζΉψ…œ ωΖ¥”Π Ϋ÷–ΒΡG «
Θ®Χν–¥Ή÷ΡΗΘ©Θ°
aΘ°NH
3 bΘ°CO
2 cΘ°SO
2 dΘ°CH
3CH
2OH
Θ®3Θ©Ρ≥―–ΨΩ–ΓΉι‘Ύ Β―ι ““‘Ag-ZSM-5ΈΣ¥ΏΜ·ΦΝΘ§≤βΒΟNOΉΣΜ·ΈΣN
2ΒΡΉΣΜ·¬ ΥφΈ¬Ε»±δΜ·«ιΩω»γΆΦ1Υυ ΨΘ°ΔΌ»τ≤Μ Ι”ΟCOΘ§Έ¬Ε»≥§Ιΐ775ΓφΘ§ΖΔœ÷NOΒΡΉΣΜ·¬ ΫΒΒΆΘ§ΤδΩ…ΡήΒΡ‘≠“ρΈΣ
ΘΜ‘Ύ
=1ΒΡΧθΦΰœ¬Θ§”ΠΩΊ÷ΤΒΡΉνΦ―Έ¬Ε»‘Ύ
Ήσ”“Θ°
ΔΎ”ΟC
xH
yΘ®ΧΰΘ©¥ΏΜ·ΜΙ‘≠“≤Ω…œϊ≥ΐΒΣ―θΜ·ΈοΒΡΈέ»ΨΘ°–¥≥ωCH
4”κNO
2ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ
Θ°
Θ®4Θ©÷ΈάμΥ°÷–œθΥα―ΈΈέ»ΨΒΡΖΫΖ® «ΘΚ
ΔΌ¥ΏΜ·Ζ¥œθΜ·Ζ®÷–Θ§”ΟH
2ΫΪNO
3-ΜΙ‘≠ΈΣN
2Θ§“ΜΕΈ ±ΦδΚσΘ§»ή“ΚΒΡΦν–‘Οςœ‘‘ω«ΩΘ°‘ρΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣΘΚ
Θ°
ΔΎ‘ΎΥα–‘ΧθΦΰœ¬Θ§ΒγΜ·―ßΫΒΫβNO
3-ΒΡ‘≠άμ»γΆΦ2Θ§Βγ‘¥’ΐΦΪΈΣΘΚ
Θ®―ΓΧνΓΑAΓ±ΜρΓΑBΓ±Θ©Θ§“θΦΪΖ¥”Π ΫΈΣΘΚ
Θ°


 ΘΜ
ΘΜ Θ°
Θ°


 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
 »γΆΦ÷–ΟΩΧθ’έœΏ±μ Ψ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΎΔτAΓΪΔςAΉε÷–ΒΡΡ≥“ΜΉε‘ΣΥΊ«βΜ·ΈοΒΡΖ–Βψ±δΜ·Θ°ΟΩΗω–ΓΚΎΒψ¥ζ±μ“Μ÷÷«βΜ·ΈοΘ§Τδ÷–aΒψ¥ζ±μΒΡ «Θ®ΓΓΓΓΘ©
»γΆΦ÷–ΟΩΧθ’έœΏ±μ Ψ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΎΔτAΓΪΔςAΉε÷–ΒΡΡ≥“ΜΉε‘ΣΥΊ«βΜ·ΈοΒΡΖ–Βψ±δΜ·Θ°ΟΩΗω–ΓΚΎΒψ¥ζ±μ“Μ÷÷«βΜ·ΈοΘ§Τδ÷–aΒψ¥ζ±μΒΡ «Θ®ΓΓΓΓΘ© ‘Ύ“ΜΕ®Έ¬Ε»œ¬Θ§»ίΤςΡΎΡ≥“ΜΖ¥”Π÷–ΝΫ÷÷ΤχΧ§Έο÷ MΓΔNΒΡΈο÷ ΒΡΝΩΥφΖ¥”Π ±Φδ±δΜ·ΒΡ«ζœΏ»γΆΦΘ§œ¬Ν–±μ ω÷–’ΐ»ΖΒΡ «Θ®ΓΓΓΓΘ©
‘Ύ“ΜΕ®Έ¬Ε»œ¬Θ§»ίΤςΡΎΡ≥“ΜΖ¥”Π÷–ΝΫ÷÷ΤχΧ§Έο÷ MΓΔNΒΡΈο÷ ΒΡΝΩΥφΖ¥”Π ±Φδ±δΜ·ΒΡ«ζœΏ»γΆΦΘ§œ¬Ν–±μ ω÷–’ΐ»ΖΒΡ «Θ®ΓΓΓΓΘ©

