̼�������仯�����ڹ�ũҵ����������������Ҫ���ã���ش��������⣺
��1����CH
4����ԭNO
x�������������������Ⱦ�����磺
CH
4��g��+4NO
2��g��=4NO��g��+CO
2��g��+2H
2O��g����H
1=-574kJ?mol
-1CH
4��g��+4NO��g��=2N
2��g��+CO
2��g��+2H
2O��g����H
2��2mol CH
4��ԭNO
2��N
2�����������зų�������Ϊ1734kJ�����H
2=
��
��2���ݱ�������ѧ����һ������������Fe
2O
3����鷴Ӧ����ȡ���������Ľ��������䷴Ӧ���£�
Fe
2O
3��s��+3CH
4��g��?2Fe��s��+3CO��g��+6H
2��g����H��0
������Ӧ��5L���ܱ������н��У�1min��ﵽƽ�⣬���Fe
2O
3�ڷ�Ӧ����������3.2g����
�ö�ʱ����CO��ƽ����Ӧ����Ϊ
��
�����÷�Ӧ�ں��º�ѹ�����н��У��ܱ����÷�Ӧ�ﵽƽ��״̬����
��ѡ����ţ�
a��CH
4��ת���ʵ���CO�IJ���
b����������ƽ����Է�����������
c��v��CO����v��H
2���ı�ֵ����
d�����������������
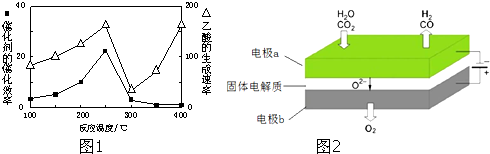
�۸÷�Ӧ�ﵽƽ��ʱij���������¶ȱ仯��ͼ1��ʾ�����¶���T
1���ߵ�T
2ʱ��ƽ�ⳣ��K
A
K
B�����������������=��������������Ա�ʾ������������Щ
��
a��H
2���淴Ӧ����
b��CH
4���������
c����������ƽ����Է�������
d��CO���������
��3����ҵ�ϳɰ�����Ҫ�ķ�Ӧ�����dz����Ҳ����ͣ���һЩ��ѧ�Ҳ��ø����ӵ����Ե�SCY�մ�
���ܴ���H
+ ��ʵ�ְ��ĵ绯ѧ�ϳɣ��Ӷ��������˵�����������ת���ʣ��绯ѧ�ϳɰ�����
���ܷ�ӦʽΪ��N
2+3H
22NH
3���ù����л�ԭ��Ӧ�ķ���ʽΪ
��
��4������20mL 0.0lmol?L
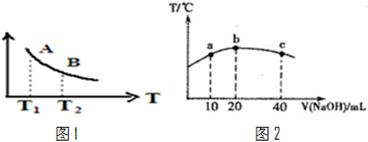
-l������HNO
2��Һ����μ���һ��Ũ�ȵ��ռ���Һ����û����Һ���¶ȱ仯��ͼ2��ʾ�������й�˵����ȷ���ǣ�������
�ٸ��ռ���Һ��Ũ��Ϊ0.02mol?L
-1�ڸ��ռ���Һ��Ũ��Ϊ0.01mol?L
-1��HNO
2�ĵ���ƽ�ⳣ����b�㣾a��
�ܴ�b�㵽c�㣬�����Һ��һֱ���ڣ�c��Na
+����c��NO
2-����c��OH
-����c��H
+��