
ŹµŃéŹŅ“Óŗ¬µā·ĻŅŗ£Ø³żH2OĶā£¬ŗ¬ÓŠCCl4”¢I2”¢I£µČ£©ÖŠ»ŲŹÕµā£¬Ę䏵Ńé¹ż³ĢČēĻĀ£ŗ
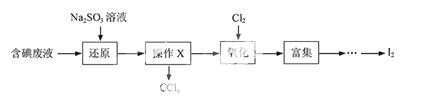
£Ø1£©Ļņŗ¬µā·ĻŅŗÖŠ¼ÓČėÉŌ¹żĮæµÄNa2SO3ČÜŅŗ£¬½«·ĻŅŗÖŠµÄI2»¹ŌĪŖI££¬ĘäĄė×Ó·½³ĢŹ½ĪŖ £»øĆ²Ł×÷½«I2»¹ŌĪŖI£µÄÄæµÄŹĒ ”£
£Ø2£©²Ł×÷XµÄĆū³ĘĪŖ ”£
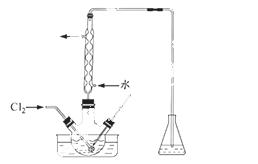
£Ø3£©Ńõ»ÆŹ±£¬ŌŚČż¾±ÉÕĘæÖŠ½«ŗ¬I£µÄĖ®ČÜŅŗÓĆŃĪĖįµ÷ÖĮpHŌ¼ĪŖ2£¬»ŗĀżĶØČėCl2£¬ŌŚ400C×óÓŅ·“Ó¦£ØŹµŃé×°ÖĆČēĶ¼ĖłŹ¾£©”£ŹµŃéæŲÖĘŌŚ½ĻµĶĪĀ¶ČĻĀ½ųŠŠµÄŌŅņŹĒ £»×¶ŠĪĘæĄļŹ¢·ÅµÄČÜŅŗĪŖ ”£
£Ø4£©ŅŃÖŖ£ŗ5SO32—+2IO3—+2H£« I2+5SO42—+H2O
I2+5SO42—+H2O
ijŗ¬µā·ĻĖ®£ØpHŌ¼ĪŖ8£©ÖŠŅ»¶Ø“ęŌŚI2£¬æÉÄÜ“ęŌŚI£”¢IO3—ÖŠµÄŅ»ÖÖ»ņĮ½ÖÖ”£Ēė²¹³äĶźÕū¼ģŃéŗ¬µā·ĻĖ®ÖŠŹĒ·ńŗ¬ÓŠI£”¢IO3—µÄŹµŃé·½°ø£ŗČ”ŹŹĮæŗ¬µā·ĻĖ®ÓĆCCl4¶ą“ĪŻĶČ””¢·ÖŅŗ£¬Ö±µ½Ė®²ćÓƵķ·ŪČÜŅŗ¼ģŃé²»³öµāµ„ÖŹ“ęŌŚ£» ”£
ŹµŃéÖŠæɹ©Ń”ŌńµÄŹŌ¼Į£ŗĻ”ŃĪĖį”¢µķ·ŪČÜŅŗ”¢FeCl3ČÜŅŗ”¢Na2SO3ČÜŅŗ
 Š”ѧ¶į¹ŚAB¾ķĻµĮŠ“š°ø
Š”ѧ¶į¹ŚAB¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«Ņ»¶ØĮæµÄ°±»ł¼×Ėįļ§¹ĢĢåÖĆÓŚÄ³ČŻ»żŗć¶ØµÄÕęæÕČŻĘ÷ÖŠ£¬·¢Éś·“Ó¦£ŗH2NCOONH4(s)2NH3(g)£«
CO2(g)£¬ŌŚ²»Ķ¬ĪĀ¶ČĻĀ£¬øĆ·“Ó¦“ļĘ½ŗāדĢ¬Ź±µÄ²æ·ÖŹż¾ŻČē±ķĖłŹ¾”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ (””””)
| ĪĀ¶Č | Ę½ŗāÅضČ(mol”¤L£1) | |
| c(NH3) | c(CO2) | |
| T1 | 0.1 | |
| T2 | 0.1 |
A£®ČōT2>T1£¬ŌņøĆ·“Ó¦µÄ¦¤H<0
B£®ĻņČŻĘ÷ÖŠ³äČėN2£¬H2NCOONH4ÖŹĮæŌö¼Ó
C£®NH3Ģå»ż·ÖŹż²»±äŹ±£¬ĖµĆ÷øĆ·“Ó¦“ļµ½Ę½ŗā
D£®T1”¢T2Ź±£¬×Ŗ»ÆµÄH2NCOONH4µÄĪļÖŹµÄĮ榤n(T2)£½2¦¤n(T1)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚŗćČŻ¾ųČČ(²»ÓėĶā½ē½»»»ÄÜĮæ)Ģõ¼žĻĀ½ųŠŠ2A(g)£«B(g)2C(g)£«D(s)·“Ó¦£¬
°“ĻĀ±ķŹż¾ŻĶ¶ĮĻ£¬·“Ó¦“ļµ½Ę½ŗāדĢ¬£¬²āµĆĢåĻµŃ¹ĒæÉżøß”£¼ņŹöøĆ·“Ó¦µÄĘ½ŗā³£ŹżÓėĪĀ¶ČµÄ±ä»Æ¹ŲĻµ£ŗ
_______________________________ӣ
| ĪļÖŹ | A | B | C | D |
| ĘšŹ¼Ķ¶ĮĻ/mol | 2 | 1 | 2 | 0 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚŗćĪĀŹ±£¬ĻņijĆܱÕČŻĘ÷ÖŠĶØČė2 mol XŗĶ1 mol YĘųĢ壬·¢ÉśČēĻĀ·“Ó¦ £ŗ2X(g)£«Y(g)2Z(g)£¬
£ŗ2X(g)£«Y(g)2Z(g)£¬
Ń¹ĒæŅ»¶ØŹ±£¬²āµĆŌŚĘ½ŗāŹ±ZµÄĢå»ż·ÖŹżĪŖ0.4”£
£Ø1£©ÓėÉĻŹöĘ½ŗā±£³ÖĶ¬ĪĀ”¢Ķ¬Ń¹£¬ČōĻņĆܱÕČŻĘ÷ÖŠĶØČė4 mol X(g)ŗĶ2 mol Y(g)£¬“ļµ½Ę½ŗā£¬ŌņZµÄĢå»ż·Ö ŹżĪŖ__________£»Ę½ŗāŹ±£¬ĘųĢåµÄ×ÜĪļÖŹµÄĮæŹĒ__________”£
ŹżĪŖ__________£»Ę½ŗāŹ±£¬ĘųĢåµÄ×ÜĪļÖŹµÄĮæŹĒ__________”£
£Ø2£©ÓėÉĻŹöĘ½ŗā±£³ÖĶ¬ĪĀ”¢Ķ¬Ń¹£¬ČōĻņĆܱÕČŻĘ÷ÖŠĶØČėX(g)ŗĶY(g)µÄĪļÖŹµÄĮæ·Ö±š¼ĒĪŖn(X)”¢n(Y)£¬²¢Āś×ćĘ½ŗāŹ±ZµÄĢå»ż·ÖŹżĪŖ0.4£¬Ōņ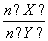 µÄȔֵ·¶Ī§ĪŖ__________”£
µÄȔֵ·¶Ī§ĪŖ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹¤ŅµÉś²ś“æ¼īµÄ¹¤ŅÕĮ÷³ĢŹ¾ŅāĶ¼ČēĻĀ£ŗ
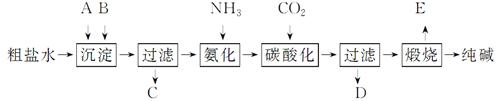
Ķź³ÉĻĀĮŠĢīæÕ£ŗ
£Ø1£©“ÖŃĪĖ®¼ÓČė³Įµķ¼ĮA”¢B³żŌÓÖŹ£Ø³Įµķ¼ĮAĄ“Ō“ÓŚŹÆ»ŅŅ¤³§£©£¬Š“³öA”¢BµÄ»ÆѧŹ½”£
A””””””£¬B”””””””””£
£Ø2£©ŹµŃéŹŅĢį“æ“ÖŃĪµÄŹµŃé²Ł×÷ŅĄ“ĪĪŖ£ŗ
Ȕѳ”¢”””””¢³Įµķ”¢”””””¢”””””¢ĄäČ“½į¾§”¢”””””¢ŗęøÉ”£
£Ø3£©¹¤ŅµÉś²ś“æ¼ī¹¤ŅÕĮ÷³ĢÖŠ£¬Ģ¼Ėį»ÆŹ±²śÉśµÄĻÖĻóŹĒ______”£Ģ¼Ėį»ÆŹ±Ć»ÓŠĪö³öĢ¼ĖįÄĘ¾§Ģ壬ĘäŌŅņŹĒ______”£
£Ø4£©Ģ¼Ėį»Æŗó¹żĀĖ£¬ĀĖŅŗD×īÖ÷ŅŖµÄ³É·ÖŹĒ_____
£ØĢīŠ“»ÆѧŹ½£©£¬¼ģŃéÕāŅ»³É·ÖµÄŅõĄė×ӵľßĢå·½·ØŹĒ____”£
£Ø5£©°±¼ī·ØĮ÷³ĢÖŠ°±ŹĒŃ»·Ź¹ÓĆµÄ£¬ĪŖ“Ė£¬ĀĖŅŗD¼ÓČėŹÆ»ŅĖ®²śÉś°±”£¼ÓŹÆ»ŅĖ®ŗóĖł·¢ÉśµÄ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ”””””””£ĀĖŅŗD¼ÓŹÆ»ŅĖ®Ē°ĻČŅŖ¼ÓČČ£¬ŌŅņŹĒ”””””””£
£Ø6£©²śĘ·“æ¼īÖŠŗ¬ÓŠĢ¼ ĖįĒāÄĘ”£Čē¹ūÓĆ¼ÓČČ·Ö½āµÄ·½·Ø²ā¶Ø“æ¼īÖŠĢ¼ĖįĒāÄʵÄÖŹĮæ·ÖŹż£¬“æ¼īÖŠĢ¼ĖįĒāÄʵÄÖŹĮæ·ÖŹżæɱķŹ¾ĪŖ£ŗ________£Ø×¢Ć÷ÄćµÄ±ķ“ļŹ½ÖŠĖłÓƵÄÓŠ¹Ų·ūŗĻµÄŗ¬Ņ壩”£
ĖįĒāÄĘ”£Čē¹ūÓĆ¼ÓČČ·Ö½āµÄ·½·Ø²ā¶Ø“æ¼īÖŠĢ¼ĖįĒāÄʵÄÖŹĮæ·ÖŹż£¬“æ¼īÖŠĢ¼ĖįĒāÄʵÄÖŹĮæ·ÖŹżæɱķŹ¾ĪŖ£ŗ________£Ø×¢Ć÷ÄćµÄ±ķ“ļŹ½ÖŠĖłÓƵÄÓŠ¹Ų·ūŗĻµÄŗ¬Ņ壩”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Na2S2O3ŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬Ņ×ČÜÓŚĖ®”£ŌŚÖŠŠŌ»ņ¼īŠŌ»·¾³ÖŠĪČ¶Ø”£
I£®ÖʱøNa2S2O3•5H2O
·“Ó¦ŌĄķ£ŗNa2SO3£Øaq£©+S£Øs£©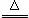 Na2S2O3(aq)
Na2S2O3(aq)
ŹµŃé²½Öč£ŗ
¢Ł³ĘČ”15g Na2SO3¼ÓČėŌ²µ×ÉÕĘæÖŠ£¬ŌŁ¼ÓČė80mlÕōĮóĖ®”£ĮķČ”5gŃŠĻøµÄĮņ·Ū£¬ÓĆ3ml ŅŅ“¼ČóŹŖ£¬¼ÓČėÉĻŹöČÜŅŗÖŠ”£
¢Ś°²×°ŹµŃé×°ÖĆ£ØČēĶ¼ĖłŹ¾£¬²æ·Ö¼Ó³Ö×°ÖĆĀŌČ„£©£¬Ė®Ō”¼ÓČČ£¬Ī¢·Š60·ÖÖÓ”£
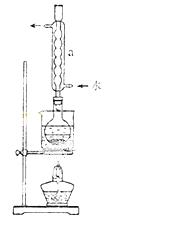
¢Ū³ĆČČ¹żĀĖ£¬½«ĀĖŅŗĖ®Ō”¼ÓČČÅØĖõ£¬ĄäČ“Īö³öNa2S2O3•5H2O£¬¾¹żĀĖ£¬Ļ“µÓ£¬øÉŌļ£¬µĆµ½²śĘ·”£
»Ų“šĪŹĢā£ŗ
£Ø1£©Įņ·ŪŌŚ·“Ó¦Ē°ÓĆŅŅ“¼ČóŹŖµÄÄæµÄŹĒ ”£
£Ø2£©ŅĒĘ÷aµÄĆū³ĘŹĒ £¬Ęä×÷ÓĆŹĒ ”£
£Ø3£©²śĘ·ÖŠ³żĮĖÓŠĪ“·“Ó¦µÄNa2SO3Ķā£¬×īæÉÄÜ“ęŌŚµÄĪŽ»śŌÓÖŹŹĒ £¬¼ģŃéŹĒ·ń“ęŌŚøĆŌÓÖŹµÄ·½·ØŹĒ ”£
£Ø4£©øĆŹµŃéŅ»°ćæŲÖĘŌŚ¼īŠŌ»·¾³ĻĀ½ųŠŠ£¬·ńŌņ²śĘ··¢»Ę£¬ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾ĘäŌŅņ£ŗ
ӣ
II.²ā¶Ø²śĘ·“æ¶Č
×¼Č·³ĘČ”Wg²śĘ·£¬ÓĆŹŹĮæÕōĮóĖ®Čܽā£¬ŅŌµķ·Ū×÷ÖøŹ¾¼Į£¬ÓĆ0.1000 mol•L‾1µāµÄ±ź×¼ČÜŅŗµĪ¶Ø”£
·“Ó¦ŌĄķĪŖ£ŗ2S2O32‾+I2=S4O62-+2I‾
£Ø5£©µĪ¶ØÖĮÖÕµćŹ±£¬ČÜŅŗŃÕÉ«µÄ±ä»Æ£ŗ ”£
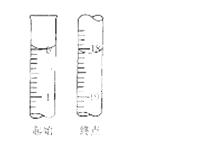
£Ø6£©µĪ¶ØĘšŹ¼ŗĶÖÕµćµÄŅŗĆęĪ»ÖĆČēĶ¼£¬ŌņĻūŗĵāµÄ±ź×¼ČÜŅŗĢå»żĪŖ mL”£²śĘ·µÄ“æ¶ČĪŖ£ØÉčNa2S2O3•5H2OĻą¶Ō·Ö×ÓÖŹĮæĪŖM£© ”£
III.Na2S2O3µÄÓ¦ÓĆ
£Ø7£©Na2S2O3»¹ŌŠŌ½ĻĒ棬ŌŚČÜŅŗÖŠŅ×±»Cl2Ńõ»Æ³ÉSO42‾£¬³£ÓĆ×÷ĶŃŃõ¼Į£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŹµŃé·½°øæÉŠŠµÄŹĒ(””””)
A£®¼ģŃéČÜŅŗÖŠŹĒ·ńÓŠSO42£Ź±£¬¼ÓČėBaCl2ČÜŅŗ£¬ŌŁ¼ÓĻ”ĻõĖį£¬¹Ū²ģ³ĮµķŹĒ·ńĻūŹ§
B£®½«ŅŅ“¼ÓėÅØĮņĖį¹²ČČÖʵƵÄĘųĢåĶØČėĖįŠŌKMnO4ČÜŅŗÖŠ£¬¼ģŃéĘųĢåÖŠŹĒ·ńŗ¬ÓŠŅŅĻ©
C£®Ń”ÓĆ25 mL¼īŹ½µĪ¶Ø¹ÜĮæČ”14.80 mL 1 mol·L£1 NaOHČÜŅŗ
D£®¼ģŃéČÜŅŗÖŠŹĒ·ńŗ¬ÓŠCO32£Ź±£¬¼ÓČėĻ”ŃĪĖį£¬½«²śÉśµÄĘųĢåĶØČė³ĪĒåŹÆ»ŅĖ®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¶ŌÓŚæÉÄę·“Ó¦2AB3(g) A2(g)£«3B2(g) ; ¦¤H£¾0£¬ĻĀĮŠĶ¼ĻńÕżČ·µÄŹĒ
A2(g)£«3B2(g) ; ¦¤H£¾0£¬ĻĀĮŠĶ¼ĻńÕżČ·µÄŹĒ
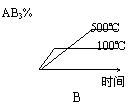
D
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ā±×åŌŖĖŲ°üĄØF”¢Cl”¢BrµČ”£
(1)ĻĀĮŠĒśĻß±ķŹ¾Ā±×åŌŖĖŲijÖÖŠŌÖŹĖęŗĖµēŗÉŹżµÄ±ä»ÆĒ÷ŹĘ£¬ÕżČ·µÄŹĒ________”£
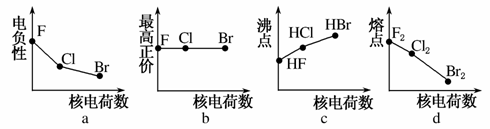
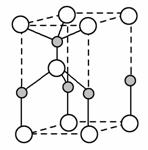
(2)ĄūÓĆ”°Ā±»ÆÅš·Ø”±æÉŗĻ³Éŗ¬BŗĶNĮ½ÖÖŌŖĖŲµÄ¹¦ÄÜĢÕ“É£¬ÓŅĶ¼ĪŖĘ侧°ū½į¹¹Ź¾ŅāĶ¼£¬ŌņĆæøö¾§°ūÖŠŗ¬ÓŠBŌ×ÓµÄøöŹżĪŖ________£¬øĆ¹¦ÄÜĢՓɵĻÆѧŹ½ĪŖ________”£
(3)BCl3ŗĶNCl3ÖŠŠÄŌ×ÓµÄŌӻƷ½Ź½·Ö±šĪŖ________ŗĶ________”£µŚŅ»µēĄėÄܼŪÓŚB”¢NÖ®¼äµÄµŚ¶žÖÜĘŚŌŖĖŲÓŠ________ÖÖ”£
(4)ČōBCl3ÓėXYnĶعżBŌ×ÓÓėXŌ×Ó¼äµÄÅäĪ»¼ü½įŗĻŠĪ³ÉÅäŗĻĪļ£¬ŌņøĆÅäŗĻĪļÖŠĢį¹©¹Ā¶Ōµē×ÓµÄŌ×ÓŹĒ________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com