
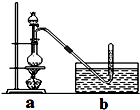
 CH3ClΪ��ɫ���Դ���ζ�����壬�ܶ�Ϊ2.25g/L���۵�Ϊ-24.2�棬20��ʱ��ˮ�е��ܽ��Ϊ400mL���������Ҵ��ͱ������л��ܼ���
CH3ClΪ��ɫ���Դ���ζ�����壬�ܶ�Ϊ2.25g/L���۵�Ϊ-24.2�棬20��ʱ��ˮ�е��ܽ��Ϊ400mL���������Ҵ��ͱ������л��ܼ������� ���������ü״���Ũ���ᷴӦ�Ʊ�CH3Cl���漰������Ʊ����������ڸ����HCl�����м���ZnCl2����õ���ˮZnCl2���ٽ���ˮZnCl2����ȡ20mLŨ�������Բ����ƿ���μ�һ�����ļ״��������Ⱥ����CH3Cl���ɣ���CH3Cl�л��лӷ�����HCl���״���ѡ����ˮ���ռ����ɵ������ͬʱ�����Գ��ӣ����̽���˵��ȶ��Լ�����CH3Clβ���Ĵ�����
��1����ZnCl2����ֱ�Ӽ���ʱ����Zn2+ˮ�⣬���ֱ�Ӽ������յõ�����Zn��OH��2�����Ҫ��Ũ�����HCl����������ˮ���ͬʱ���ȣ�
��2���������ַ�Ӧ��μӷ�Ӧʱ����ͨ�����һ�ַ�Ӧ����������һ�ַ�Ӧ���ת���ʣ�
��3����Ӧ������е�����ͼ״����ӷ������ɵ�CH3Cl�л���лӷ��������ʣ�ͬʱCH3Cl������ˮ��ѡ����ˮ���ռ���ͬʱ�����г��ӵ����ã�
��1�����CH3Cl�ܱ����Ը�����������������Ը��������Һ��������ɫ��ͬʱ���ܻ�����CO2��Cl2�����Ҫע��β���Ĵ�����
��2��CH3Cl�������Ը�����ص�ϴ��ƿ�ᱻ������CO2��Cl2�����ɵ�Cl2��ѡ��Na2SO3��Һ�����������������ԣ���Na2SO3����ΪNa2SO4��
��3����CH3Cl�������Ҵ��ͱ������л��ܼ�����˵�β����ѡ���л��ܼ����գ�
��� �⣺��1���ڼ���ZnCl2����ʱ��ѡ��ͨHCl����������ˮ�⣬�ʴ�Ϊ���ڸ����HCl�����м��ȣ�
��2��Ϊ����״��������ʣ������ʵ���������������ʴ�Ϊ�����������������״���ת���ʣ�
��3����CH3Cl�л���������ˮ���������壬����ˮ�����Լ������ֿ��ռ����ʴ�Ϊ��CH3Cl��������ˮ������ˮ���ɳ�ȥHCl��CH3OH���������壻
��1�����CH3Cl�ܱ�ǿ���������Ը��������������ѡ����ͨ��ʢ��ˮ��ϴ��ƿ��ȥ���ܻ���е�HCl�ͼ״�����ͨ��ʢ�е����Ը������ϴ��ƿ����Һ����ɫ���ȥ�����ͨ��ʢNa2SO3��ϴ��ƿ�������ɵ���������Ⱦ���ʴ�Ϊ��BAC��A��ɫ��ȥ��
��2�����Ը����������CH3Cl������CO2��Cl2��ͬʱ��������ԭ��Mn2+��������Ӧ�����ӷ���ʽΪ10CH3Cl+14MnO4-+42H+=14Mn2++10CO2��+5Cl2��+36H2O�����ɵ���������Na2SO3��ϴ��ƿ������Na2SO3ΪNa2SO4��������Ӧ�����ӷ���ʽΪSO32-+Cl2+H2O=SO42-+2Cl-+H+���ʴ�Ϊ��10CH3Cl+14MnO4-+42H+=14Mn2++10CO2��+5Cl2��+36H2O��SO32-+Cl2+H2O=SO42-+2Cl-+H+��
��3��CH3Cl�������Ҵ��ͱ������л��ܼ�����ѡ��ƾ�����CH3Cl��β�����ʴ�Ϊ���Ҵ���
���� ���⿼���˼״���Ũ�����ϼ�����һ�ȼ����ʵ��ԭ�����漰ʵ�����������ˮ�⡢���ʵ����ʼ��鼰��Ӧԭ����̽�����ۺ��Խ�ǿ����������֪ʶƫ�࣬�ѶȲ������ӷ���ʽ����д�����ʿ���ƫ�ߵ㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������֬�ڼ��������µ�ˮ�ⷴӦ�������������ͺͷ��� | |
| B�� | ��֬��С������ø�Ĵ����ö�ˮ�⣬���ɵĸ�֬���������Ϊ����Ӫ���ɷ�Ϊ���������գ�ͬʱ�ṩ��������Ҫ������ | |
| C�� | ��֬�е�̼��Ϊ̼̼����ʱ����Ҫ�Ǹ߷е�Ķ���֬�� | |
| D�� | ��֬����������Ļ���Ӫ������֮һ��Ӧ������ʳ����֬������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ϡ��ˮ�ͷ�̪��Һ | B�� | ���з�̪��Na2CO3��Һ | ||
| C�� | ���з�̪������������Һ | D�� | ����SO2��Ʒ����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NA��N2���Ӻ�NA��CO2ԭ�Ӹ�����Ϊ1��1 | |
| B�� | 1 mol H2O��������NA��H2O�������ܺͼ������ | |
| C�� | 1mol H2������ԭ����ΪNA | |
| D�� | 1mol H2SO4��������������Ŀһ����NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʹ��̪���ɫ����Һ�У�Na+��K+��OH-��S2O${\;}_{3}^{2-}$ | |
| B�� | �ڼ��������ܲ�����������Һ�У�NH${\;}_{4}^{+}$��Fe2+��SO${\;}_{4}^{2-}$��NO${\;}_{3}^{-}$ | |
| C�� | ��������ˮ�������������Ũ��c��H+��=1��10-14mol/L����Һ�У�Ba2+��NO${\;}_{3}^{-}$��K+��F- | |
| D�� | ������Cu2+����Һ�У�NH${\;}_{4}^{+}$��Na+��Cl-��HS- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1.12L | B�� | 4.48L | C�� | 5.6L | D�� | 3.36L |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com