
KNO3����Ҫ�Ļ�����Ʒ��������һ���ѻ��ר����KNO3�Ʊ���������Ҫ���裺
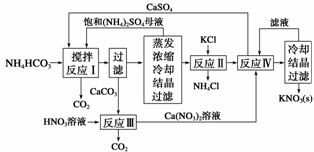
(1)��Ӧ���У�CaSO4��NH4HCO3�����ʵ���֮��Ϊ1��2���÷�Ӧ�Ļ�ѧ����ʽΪ_____
___________________________________________________________________��
(2)��Ӧ�����ڸ�̬�����ȵ������½��У����ȵ�Ŀ����___________________________
___________________________________ _____________________________________��
_____________________________________��
�ӷ�Ӧ�����û�����з����CaSO4�ķ����dz��ȹ��ˣ����ȹ��˵�Ŀ����________________________________________________________________________��
(3)���鷴Ӧ������K2SO4���Ƿ����KCl�ķ����ǣ�ȡ����K2SO4��Ʒ�ܽ��� ˮ��________________________________________________________________________��
ˮ��________________________________________________________________________��
(4)���������У���ѭ�����õ����ʳ�(NH4)2SO4�⣬����________(�ѧʽ)��
 ��У����ϵ�д�
��У����ϵ�д�
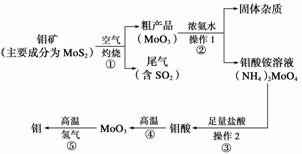
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и��ѧ�����б�ʾ��ȷ���ǣ� ��
A��Na2O2�ĵ���ʽΪ: 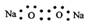
B��������ӵı���ģ��Ϊ��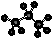
C����ȩ�Ľṹ��ʽ��HCHO
D���۱�ϩ�Ľṹ��ʽΪ�� 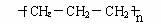
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ������������ء�����˵������ȷ����
A����ϩ����ˮ���Ĵ���� B�����Ϳ����ڻ�����Ʒ
C���Ͼƿ��Գ�ȥʳ���е���ζ D���ѻ����Ϳ�������ȡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ָ����Ӧ�����ӷ���ʽ��ȷ����
A����CH3COOH�ܽ�CaCO3��CaCO3��2H��===Ca2����H2O��CO2��
B��Cu����ϡ����HNO3��Cu��2H����NO3��===Cu2����NO2����H2O
C��(NH4)2Fe(SO4)2��Һ�����NaOH��Һ��Ӧ��Fe(OH)2��Fe2����2OH��===Fe(OH)2��
D����NaAlO2��Һ��ͨ�����CO2��CO2��AlO2����2H2O===Al(OH)3����HCO3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Q��R��X��Y��ZΪ���ڱ���ԭ���������ε�����ǰ������Ԫ�ء�
��֪����QΪԪ�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ���R�Ļ�̬ԭ���е���ռ������������ͬ���ܼ�����ÿ���ܼ��еĵ���������ͬ����Y�Ļ�̬ԭ�ӵĺ���ɶԵĵ�������δ�ɶԵĵ�������3������Z��̬ԭ��������δ�ɶԵ��ӡ��ö�Ӧ��Ԫ�ط��Ż�ѧʽ�ش��������⣺
(1)R��X��Y�ĵ�һ�������ɴ�С��˳��Ϊ ��
(2)QԪ����Ԫ�����ڱ������� ������YԪ��ͬ��ĵ�������Ԫ�صļ۵����Ų�ʽΪ ��
(3)RY2�ĵ���ʽΪ ��Z3+��ԭ�ӽṹʾ��ͼΪ____________��
(4)RQ2Y2�ĽṹʽΪ ��m g��������ȫȼ�պ�ͨ������Na2O2�������գ�Na2O2�������ص�����Ϊ g��
(5)��Y��Z����Ԫ����ɵ�Z2Y72�����������������¿����Ҵ���Ӧ���������ᡢZ3+�����ʡ��÷�Ӧ�����ӷ���ʽΪ__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ�ֹ��ɽ���Ԫ�أ�ͨ�������Ͻ���ֵ����Ӽ�������Ԫ�ؿ���ǿ�Ͻ��ǿ�ȡ�Ӳ�ȡ��ɺ��Լ����ԣ�������ǿ�����¼���ʴ���ܡ���ͼ�ǻ����������Ʊ����������Ҫ����ͼ��
(1)д����Ӧ�ٵĻ�ѧ����ʽ��___________________________________________��
(2)��Ӧ�ٵ�β�����������ã�д��Ӧ�ø�β���Ƶõ�������Ҫ��ѧ�Լ�________________________________________________________________________��
(3)�����ʵ����ģ�����1�Ͳ���2������Ҫʹ�õ���Ҫ����������________________________________________________________________________��
(4)���ڿ��������������������⣬����������������������Һ���������ƣ��������ⲻ���������ϡ���ᡣ�����ƵĻ�ѧʽΪ________��
(5)��ҵ���Ʊ���ԭ������CO��H2�ķ�Ӧԭ��ΪCO2��CH4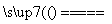 2CO��2H2��CH4��H2O
2CO��2H2��CH4��H2O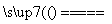 CO��3H2���������������Ϊ80%��a L(��״��)��Ȼ��������������̼��ˮ�����Ļ�����ڸ����·�Ӧ������ת����Ϊ90%���ò����Ļ�ԭ������(CO��H2)��ԭMoO3���⣬�������������������Ϊ________(ͭ�����ԭ������Ϊ96)��
CO��3H2���������������Ϊ80%��a L(��״��)��Ȼ��������������̼��ˮ�����Ļ�����ڸ����·�Ӧ������ת����Ϊ90%���ò����Ļ�ԭ������(CO��H2)��ԭMoO3���⣬�������������������Ϊ________(ͭ�����ԭ������Ϊ96)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���б����и����ȷ��һ����
| ��� ѡ�� | �� | �� | ����� | �ǵ���� |
| A | �ռ� | С�մ� | BaSO4 | �ɱ� |
| B | ���� | CaCO3 | NH3��H20 | Fe |
| C | ���� | ʳ�� | ʯī | ���� |
| D | KOH | CuSO4 | Na2SO4 | NaClO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ѧ��ѧ�г������ʵ�ת����ϵͼ�У���Ӧ���������ַ�Ӧ��Ͳ���δȫ��ע������֪A��DΪ�ճ������г����Ľ������ʣ�������Ϊ��������BΪ����ɫ���塣��ش��������⣺21���ͽ�������Ȩ����
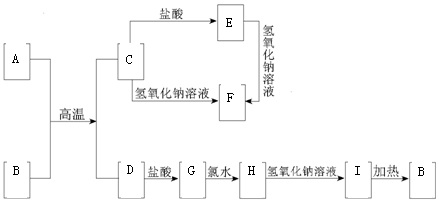 |
��1��д��A��ԭ�ӽṹʾ��ͼ
��2��д���������ʵĻ�ѧʽ�� B�� E��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
C��F�Ļ�ѧ����ʽ
G��H�����ӷ���ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����
A��������ˮ��Һ�д��ڵ������4��
B��H��O������Ϊ463 kJ��mol-1����18 g ��̬ˮ�ֽ��H2��O2ʱ,��������Ϊ2��463 kJ
C������ĥ����þ������Ƭ�ֱ����ˮ���ã��������̪��Һ���۲�����ɱȽ�26Al��26Mg������ǿ��
D�������£�V1 L pH=3��������V2LpH=11��NaOH��Һ��Һ��ϣ����Է��Ӽ��϶���������仯������Ϻ���ҺpH=4����V1��V2=11��9
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com