
����Ŀ������������һ����Ҫ�Ļ���ԭ�ϣ������Ʊ�һϵ������(����ͼ)������˵���������( )

A. ��ʽ������ˮ���ܲ���Fe(OH)3���壬��������ˮ��
B. Ϊ��ֹNH4HCO3�ֽ⣬����FeCO3���ڽϵ��¶��½��У��÷�Ӧ�����ӷ���ʽΪ��Fe2++2HCO3��= FeCO3+ CO2��+H2O
C. ����KSCN��Һ����(NH4)2Fe(SO4)2�Ƿ�����
D. ���¶��£�(NH4)2Fe(SO4)2��ˮ�е��ܽ�ȱ�FeSO4�Ĵ�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����2CH3CHO��O2![]() 2CH3COOH������AΪ��Ҫԭ�Ϻϳɻ�����E����ϳ�·����ͼ1��ʾ���ش��������⣺
2CH3COOH������AΪ��Ҫԭ�Ϻϳɻ�����E����ϳ�·����ͼ1��ʾ���ش��������⣺
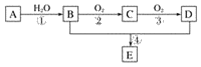
ͼ1
(1)д���������ʵĹ��������ƣ�
B��____________________��D��____________________��
(2)��Ӧ�ܵĻ�ѧ����ʽΪ________________________________________________����Ӧ���ͣ�________��
(3)ijѧϰС���������B��������ʵ��װ�����£�����ͼ2װ�ûش����⡣
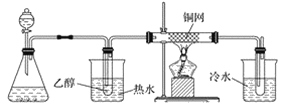
�� �� �� ��
ͼ2
��װ�ü���ƿ��ʢ�ŵĹ���ҩƷ����Ϊ________(����ĸ)��
A Na2O2 B KCl C Na2CO3 D MnO2
��ʵ������У���װ��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽΪ_________________________��
������B�Ĵ����������������Ǿ�����ͬ��������Ӧ�������õ���������μӵ�����������ͭ����Һ�м��ȣ�����Ϊ______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E�Ǻ˵����������������ֶ���������Ԫ�أ�Aԭ�Ӻ���ֻ��1�����ӣ�Bԭ�Ӱ뾶����������������С�ģ�B������������Ӧ��ˮ����Ļ�ѧʽΪHBO3��Cԭ�������ĵ������ȴ����Ķ�4��C�ļ���������D�������Ӿ�����ͬ�ĵ��Ӳ�ṹ����Ԫ�ؿ��γɻ�����D2C��C��Eͬ���塣�ش��������⣺
(1)B��Ԫ�����ڱ��е�λ��Ϊ______________________��
(2)E���������Ӧ��ˮ������______________________ (д��ѧʽ)��
(3)B��C��D��E�γɵļ����Ӱ뾶�ɴ�С�Ĺ�ϵ��______________________(�����ӷ��ű�ʾ)��
(4)������D2C���γɹ���Ϊ______________________(�õ���ʽ��ʾ)��
(5)��A��B��C����Ԫ���γɵij��������ӻ�����Ļ�ѧʽΪ___________���û������ˮ��Һ��ǿ����Һ���ȷ�����Ӧ�����ӷ���ʽΪ______________________��
(6)������D2EC3��һ�������¿��Է����ֽⷴӦ���������Σ�����һ�ֲ���Ϊ�������Σ���˷�Ӧ�Ļ�ѧ����ʽΪ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��POCl3�������뵼����Ӽ������άԭ�ϣ�ʵ���Ҳ�����������Һ̬PCl3����ȡPOCl3���й����ʵIJ����������±�
���� | �۵�/�� | �е�/�� | ��Է������� | ���� |
PCl3 | -112.0 | 76.0���ӷ��� | 137.5 | �����ܣ���Ϊ��ɫҺ�壬��ˮ�����ҷ�Ӧ���ɺ�������Ȼ��� |
POC13 | 2.0 | 106.0 | 153.5 |
ʵ��װ�ã����ȼ��г������ԣ����£�
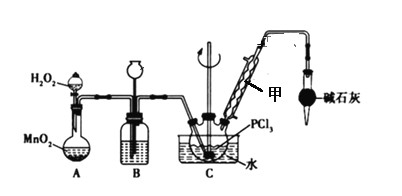
��1����װ�õ�������____________������ܵ�������____________
��2��װ��C������POCl3�Ļ�ѧ����ʽΪ______________
��3��װ��B�����ó��۲�����������⣬����________ ��________
��4����Ӧ�¶Ȳ��ܹ��ߣ�ԭ����______________��
��5����Ӧһ��ʱ�������ƿ�е�Һ����ȴ�����£�ȷ��ȡ29.1g��Ʒ������PCl3���ʣ�������ʢ��60.00 mL����ˮ���ܱ�ˮ��ƿ��ҡ������ȫ��ˮ��Ӧ����ˮ��Һ���100.00 mL��Һ������AgNO3��Һ��ǡ����ȫ��Ӧ��������ɵ�AgCl������Ϊ86.1g�����Ʒ��POC13����������Ϊ___��������λ��Ч���֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���١�����Ԫ�����ڱ��Ķ�Ӧλ����ͼ��ʾ���ش��������⡣
���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� |
��1��Ԫ�آ�ԭ�ӽṹʾ��ͼ___________��Ԫ�ص�������ۢ�___________��(��>��<��=)
��2����Ԫ�آݺ͢��γɵĻ������ˮ��Һ�м�������Ģٵ��⻯���ˮ��Һ����Ӧ�����ӷ���ʽ��______________
��3��Ԫ�آ۵�����������Ӧ��ˮ����Ϊ_______�������ӻ�����ۻ����
��4��Ԫ�آں͢��γɵ�һ�ֻ�����Ϊ����ɫ���壬�û�����ĵ���ʽΪ______���û������л�����_______�������Ӽ������Լ���Ǽ��Լ������û�������ߵ���������ﷴӦ�Ļ�ѧ����ʽΪ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵�����ʾ������ȷ������ ��
A���������������������ֱ���ȫȼ�գ����߷ų�������
B����C(ʯī)��C(���ʯ) ��H = +1.9 kJ��mol��1��֪ʯī�Ƚ��ʯ�ȶ�
C����ϡ��Һ�У�H++OH�� ![]() H2O ��H =��57.3 kJ��mol��1��������1mol CH3COOH�Ĵ�����Һ�뺬1 mol NaOH����Һ��ϣ��ų�������С��57.3 kJ
H2O ��H =��57.3 kJ��mol��1��������1mol CH3COOH�Ĵ�����Һ�뺬1 mol NaOH����Һ��ϣ��ų�������С��57.3 kJ
D�����Ȼ�ѧ����ʽ�������Ƿ�Ӧ�ﻹ���������������ۼ�״̬
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ������·���ѩ�����dz�ʹ��һ����ѩ��������Ҫ�ɷֵĻ�ѧʽΪXY2��X��Y��Ϊ���ڱ�ǰ20��Ԫ�أ��������Ӻ������ӵĵ��Ӳ�ṹ��ͬ����1 mol XY2����54mol���ӡ�
��1������ѩ���Ļ�ѧʽ��____���������л�ѧ��������______������ʽ��______��
��2��Ԫ��D��Eԭ�ӵ�����������������Ӳ�����2����D��Y���ڣ���D�����ӽṹʾ��ͼ��__________��D��E���γ�һ�ֽṹ������CO2����ԭ�ӷ��ӣ���ÿ��ԭ�Ӿ��ﵽ��8e���ȶ��ṹ���÷��ӵĽṹʽΪ_________������ʽΪ_______����ѧ������Ϊ_________(����Ӽ������Ǽ��Թ��ۼ������Թ��ۼ���)��
��3��W����Dͬ����Ķ�����Ԫ�أ�Z�ǵ������ڽ�������ǿ��Ԫ�أ�Z�ĵ�����W�ij��������з�Ӧʱ�����ֲ��������ʱ����____���仯ѧ������Ϊ___������ʱ����_____���仯ѧ������Ϊ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������A��B��C��D����ѧ�����Ļ���������ᴿ�Ļ���װ�ã�
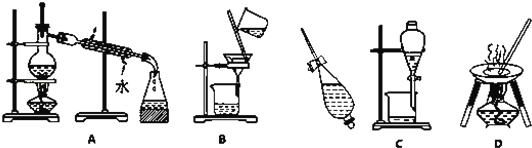
����ݻ���������ᴿ��ԭ������A��B��C��D �����ʵ��Ŀո��У�
(1)�����Ȼ�̼��ˮ����ȡ��_____��
(2)��ȥ�����е���ɳ_____��
(3)�뺣ˮɹ��ԭ�����Ƶ���_____��
(4)����CCl4(�е㣺76.75��)�ͼױ�(�е㣺110.6��)�Ļ����_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�ѧʵ�������������ȷ���ǣ� ��
A.�ӵ�ˮ����ȡI2ʱ�����ˮ��Һ����ƾ�������ȡ��Һ
B.�����ᾧʱ��������Ӧ����ʯ�����ϼ���
C.��10 mL��Ͳ��ȡ7.50 mLŨ����
D.����ʱ������ˮ���������¹ܿڽ������Ϲܿڳ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com